Surgical Site Infection Control Market Report
Published Date: 31 January 2026 | Report Code: surgical-site-infection-control
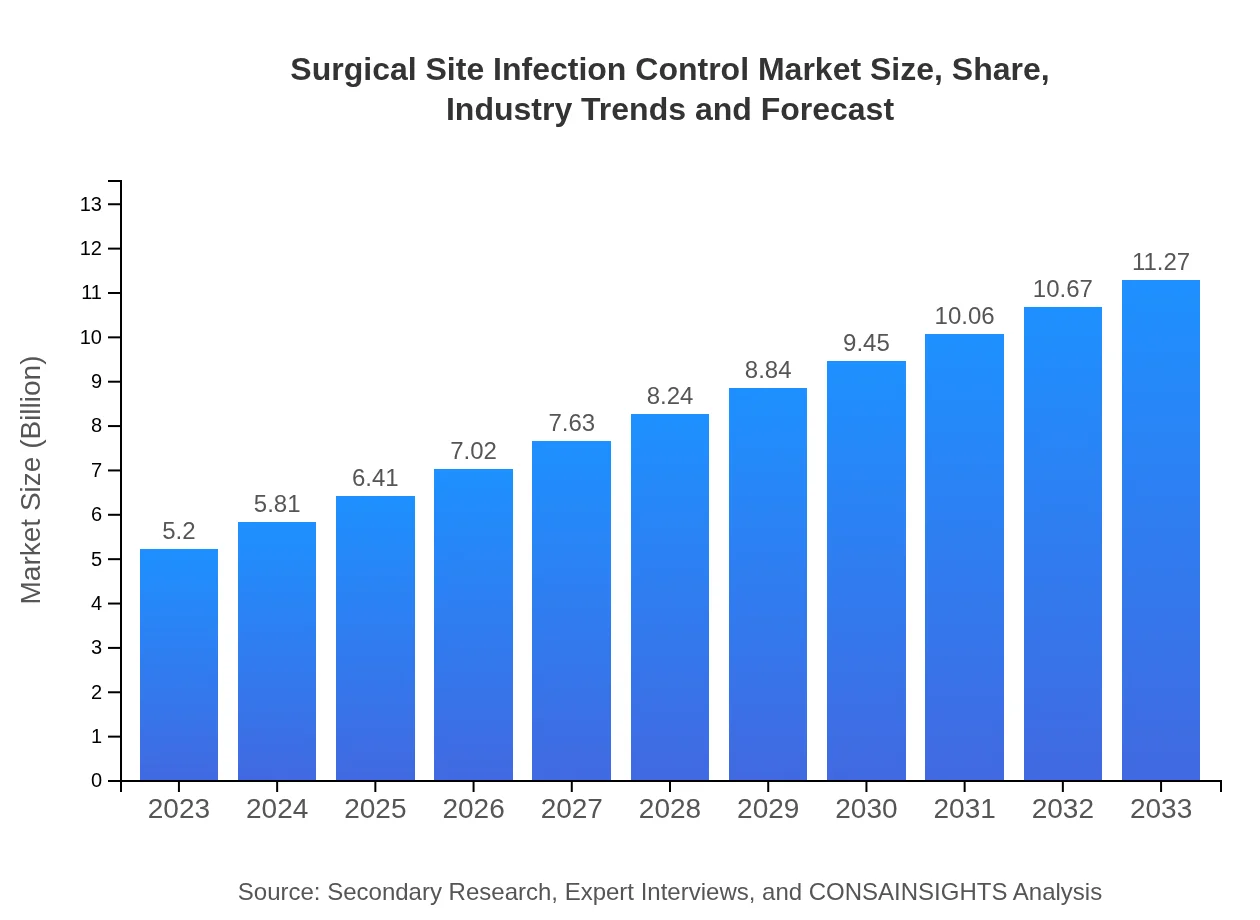
Surgical Site Infection Control Market Size, Share, Industry Trends and Forecast to 2033
This report offers a comprehensive analysis of the Surgical Site Infection Control market from 2023 to 2033. It encompasses market size, growth forecasts, segmentation, regional insights, and trends impacting the industry, providing valuable data for stakeholders and industry players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $11.27 Billion |
| Top Companies | Johnson & Johnson, 3M Health Care, Kimberly-Clark Corporation, Medline Industries, Inc. |
| Last Modified Date | 31 January 2026 |
Surgical Site Infection Control Market Overview
Customize Surgical Site Infection Control Market Report market research report
- ✔ Get in-depth analysis of Surgical Site Infection Control market size, growth, and forecasts.
- ✔ Understand Surgical Site Infection Control's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Surgical Site Infection Control
What is the Market Size & CAGR of Surgical Site Infection Control market in 2023?
Surgical Site Infection Control Industry Analysis
Surgical Site Infection Control Market Segmentation and Scope
Tell us your focus area and get a customized research report.
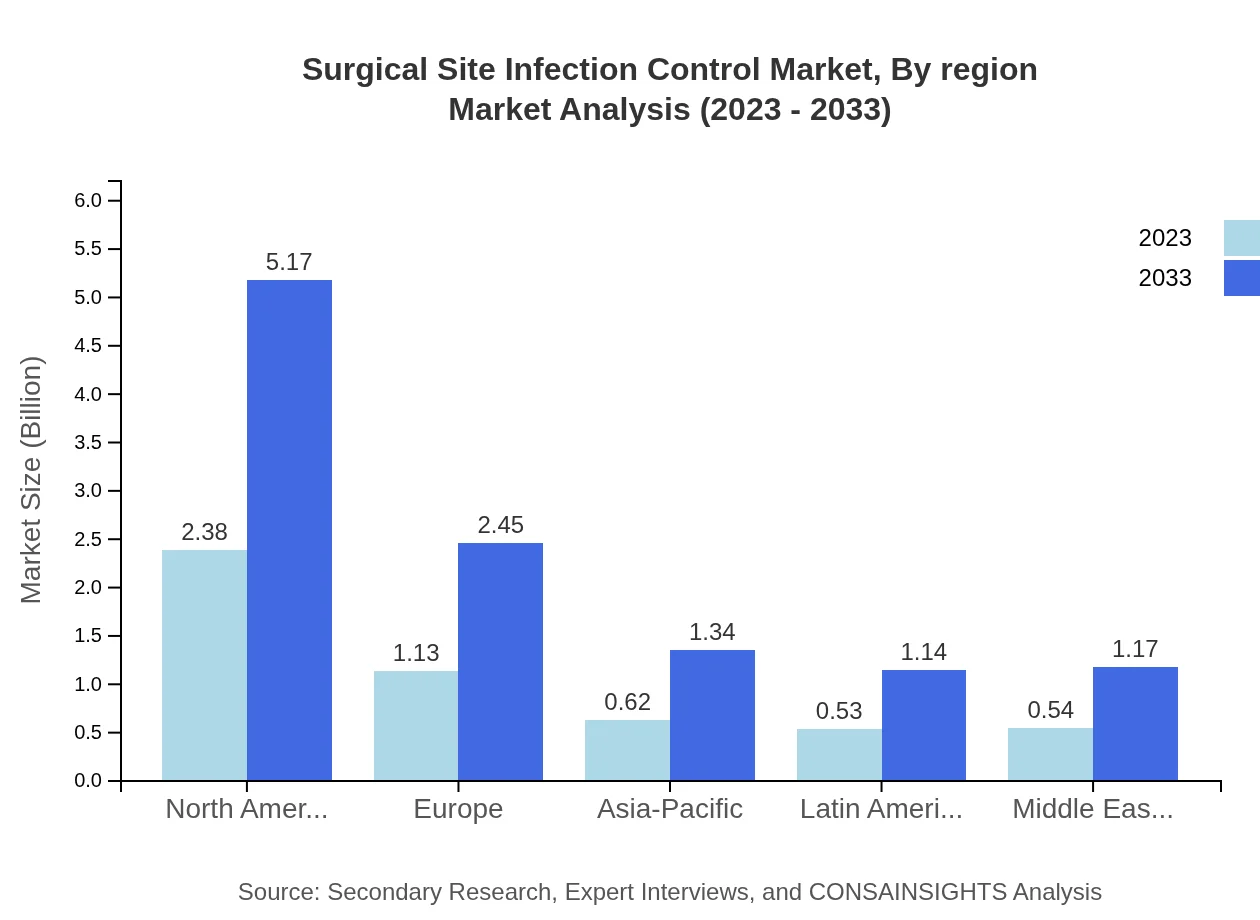
Surgical Site Infection Control Market Analysis Report by Region
Europe Surgical Site Infection Control Market Report:
Europe's Surgical Site Infection Control market stood at $1.87 billion in 2023, projected to grow to $4.05 billion by 2033. Increasing regulations for infection control in healthcare settings contribute to this growth, alongside a rising number of surgical interventions.Asia Pacific Surgical Site Infection Control Market Report:
In the Asia Pacific region, the Surgical Site Infection Control market was valued at $0.89 billion in 2023 and is projected to reach approximately $1.92 billion by 2033. The growing population and rising healthcare expenditure are key drivers of this growth, along with increasing surgical procedures in emerging economies.North America Surgical Site Infection Control Market Report:
North America currently leads the market with a value of $1.70 billion in 2023, expected to grow to $3.69 billion by 2033. The region's market growth is propelled by advanced healthcare facilities and stringent infection control regulations.South America Surgical Site Infection Control Market Report:
The South American market, with a value of $0.11 billion in 2023, is expected to grow to about $0.23 billion by 2033. Growth is facilitated by improving healthcare infrastructure and rising awareness about surgical safety in the region.Middle East & Africa Surgical Site Infection Control Market Report:
The market in the Middle East and Africa was valued at $0.64 billion in 2023, expected to grow to around $1.38 billion by 2033. The expanding healthcare sector and increasing focus on patient safety are significant growth factors.Tell us your focus area and get a customized research report.
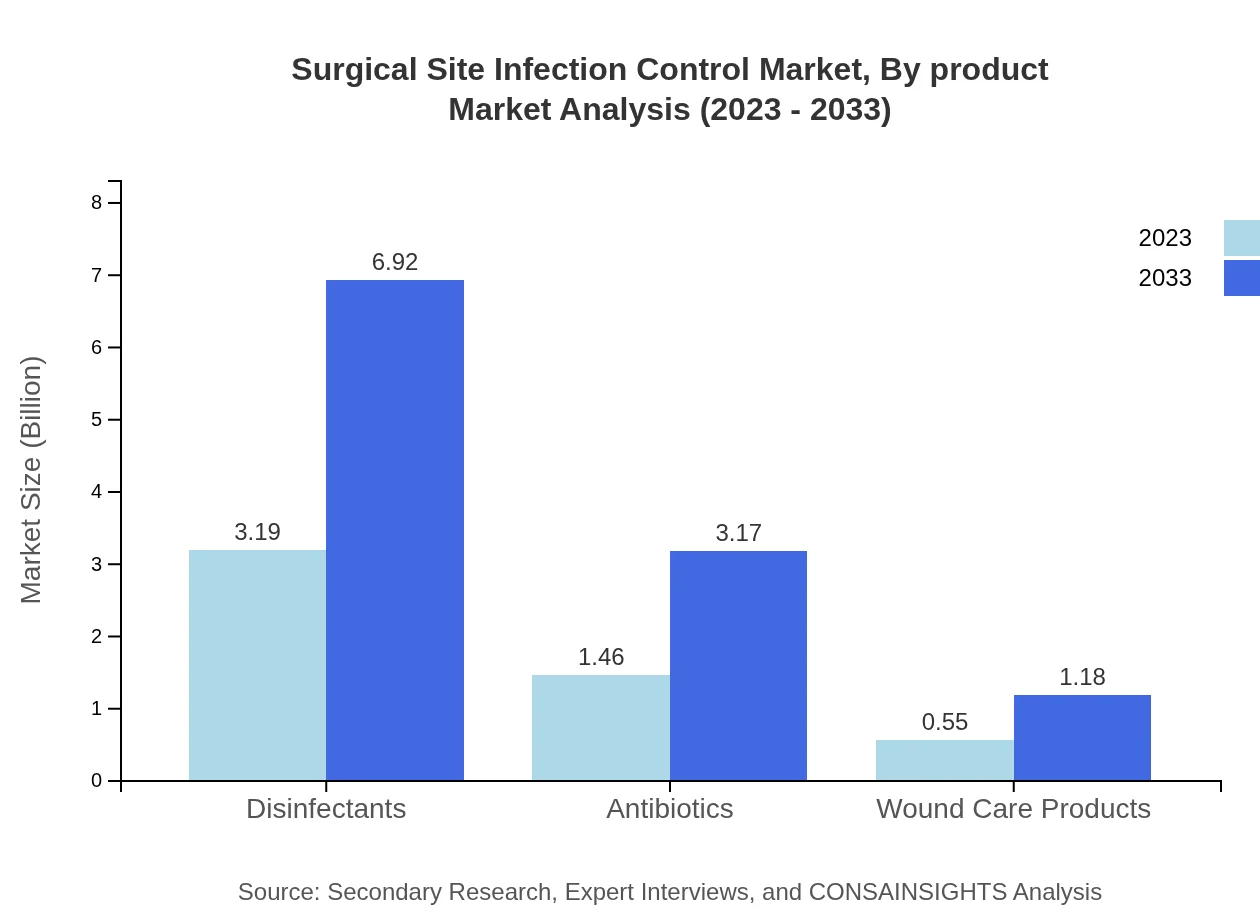
Surgical Site Infection Control Market Analysis By Product
In 2023, disinfectants lead the market with a share of 61.37% and a size of $3.19 billion, while antibiotics represent 28.13% with $1.46 billion. Wound care products account for 10.5% with $0.55 billion. By 2033, disinfectants are expected to reach $6.92 billion, maintaining the largest market share.
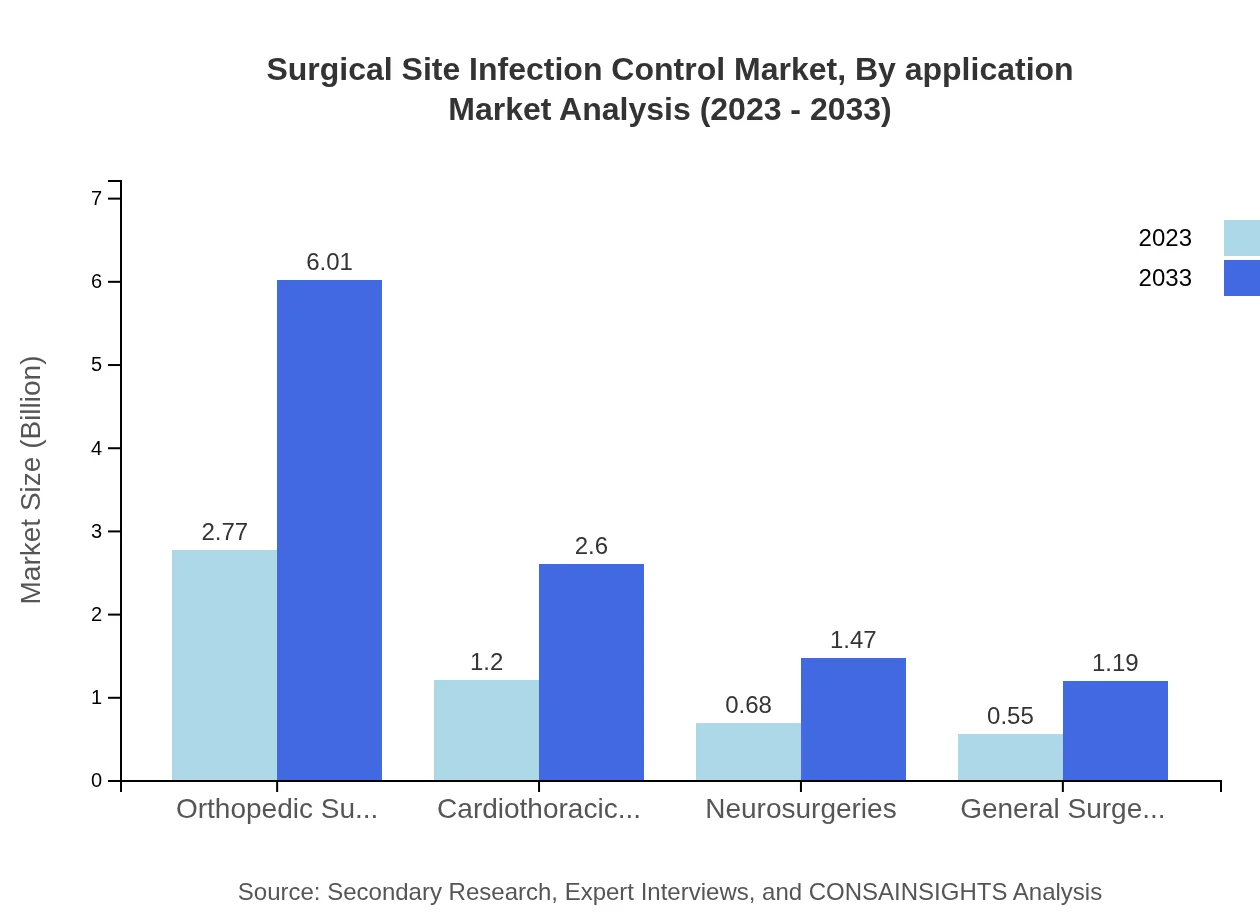
Surgical Site Infection Control Market Analysis By Application
The largest application segment is orthopedic surgeries, which commands a 53.33% market share, valued at $2.77 billion in 2023, projected to grow to $6.01 billion by 2033. Cardiothoracic surgeries account for 23.03%, followed by neurosurgeries at 13.05%, highlighting the focus on high-risk surgical procedures.
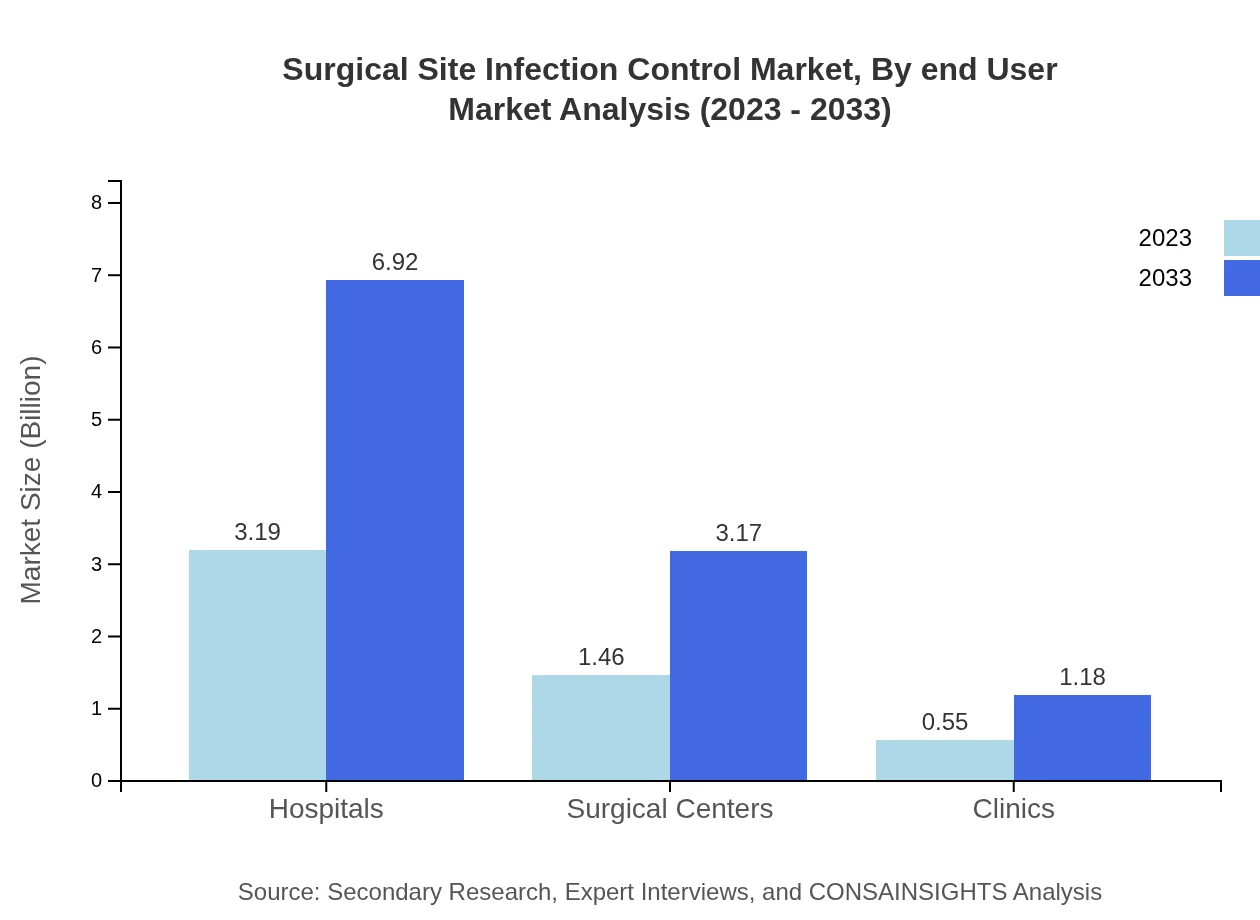
Surgical Site Infection Control Market Analysis By End User
Hospitals dominate this space, representing 61.37% of the market with a value of $3.19 billion in 2023, projected to reach $6.92 billion by 2033. Surgical centers and clinics follow, each reflecting significant market shares that contribute to the overall growth.
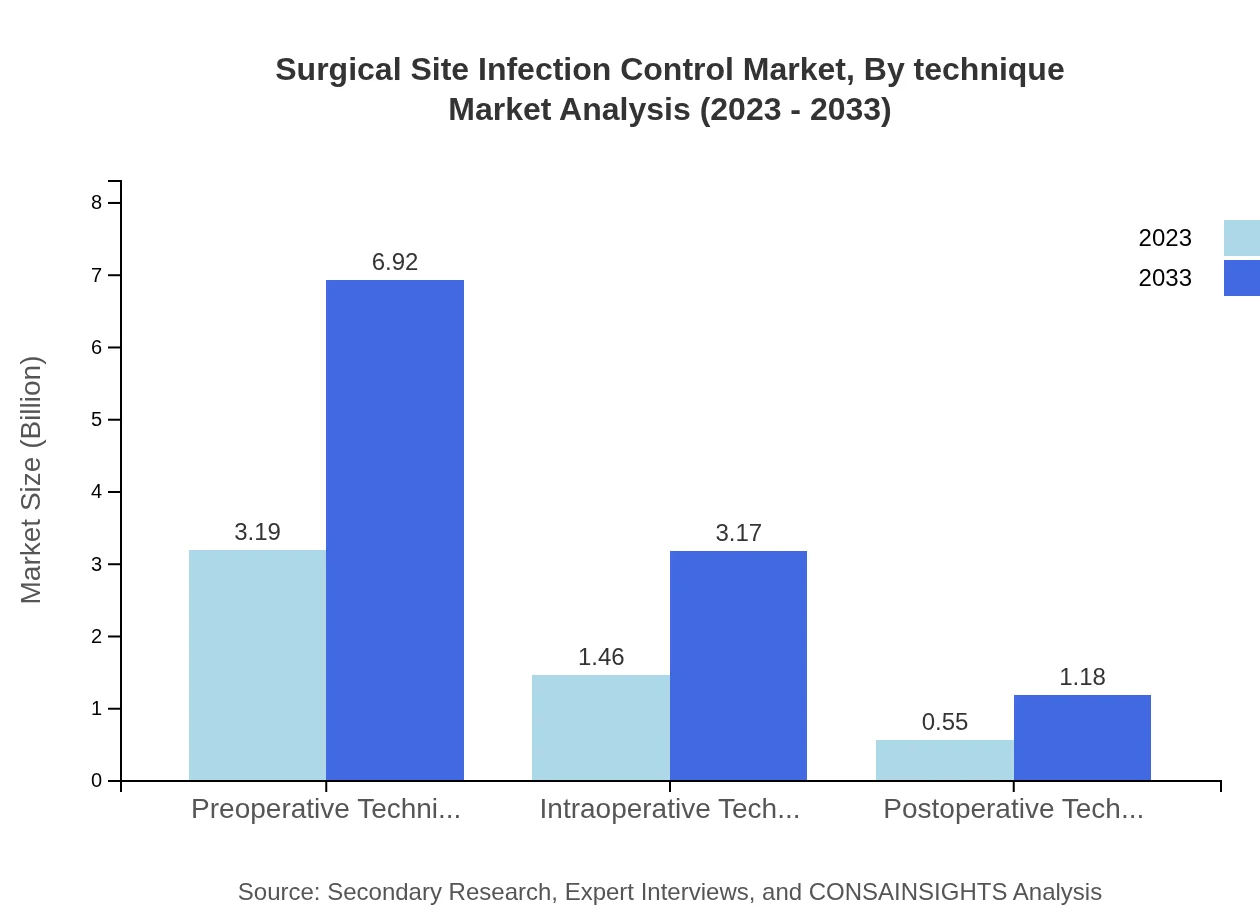
Surgical Site Infection Control Market Analysis By Technique
Preoperative techniques lead with a market share of 61.37% in 2023, valued at $3.19 billion, growing to $6.92 billion by 2033. Other techniques include intraoperative at 28.13% and postoperative at 10.5%, indicating the balanced focus on prevention during all stages of surgery.
Surgical Site Infection Control Market Analysis By Region
Regionally, North America holds the largest market share at 45.86% in 2023, with Europe at 21.75% and Asia-Pacific at 11.91%. Each region's unique healthcare challenges and solutions contribute to distinct growth trajectories.
Surgical Site Infection Control Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Surgical Site Infection Control Industry
Johnson & Johnson:
A leading player in the surgical market, Johnson & Johnson offers a wide range of surgical products, including antiseptic solutions and surgical instruments focused on infection prevention.3M Health Care:
3M Health Care provides advanced infection prevention solutions and surgical products, recognized for their innovative technologies and commitment to improving healthcare outcomes.Kimberly-Clark Corporation:
Specializing in health and hygiene products, Kimberly-Clark develops sterilization and infection control solutions critical for surgical environments.Medline Industries, Inc.:
Medline offers a diverse range of infection control products, including sterile goods and disinfectants, catering to hospitals and surgical centers.We're grateful to work with incredible clients.









FAQs
What is the market size of surgical Site Infection Control?
The surgical site infection control market is projected to reach approximately $5.2 billion by 2033, growing at a compound annual growth rate (CAGR) of 7.8% from its current value in 2023.
What are the key market players or companies in this surgical Site Infection Control industry?
Key market players in the surgical site infection control industry include major healthcare companies focusing on infection prevention, surgical products, and related pharmaceuticals. Their innovative solutions drive competition and market growth.
What are the primary factors driving the growth in the surgical Site Infection Control industry?
Growth in this industry is primarily driven by increasing surgical procedures, rising awareness of infection control, advancements in medical technology, and stringent regulatory standards aimed at enhancing patient safety.
Which region is the fastest Growing in the surgical Site Infection Control?
The Asia-Pacific region exhibits rapid growth in the surgical site infection control market, projected to expand from $0.89 billion in 2023 to $1.92 billion by 2033, reflecting a significant market potential.
Does ConsaInsights provide customized market report data for the surgical Site Infection Control industry?
Yes, ConsaInsights offers customized market report data tailored to specific business needs in the surgical site infection control industry, allowing stakeholders to make informed decisions.
What deliverables can I expect from this surgical Site Infection Control market research project?
Deliverables include comprehensive market analysis, regional insights, competitive landscape, trends analysis, and forecasts for market size and growth, tailored to your requirements.
What are the market trends of surgical Site Infection Control?
Key trends include increasing adoption of advanced disinfectants, emphasis on preoperative, intraoperative, and postoperative techniques, with hospitals leading market share, particularly in North America.