Syndromic Multiplex Diagnostic Market Report
Published Date: 31 January 2026 | Report Code: syndromic-multiplex-diagnostic
Syndromic Multiplex Diagnostic Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Syndromic Multiplex Diagnostic market, including insights on market size, growth trends, segmentation, technology advancements, and regional performance from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
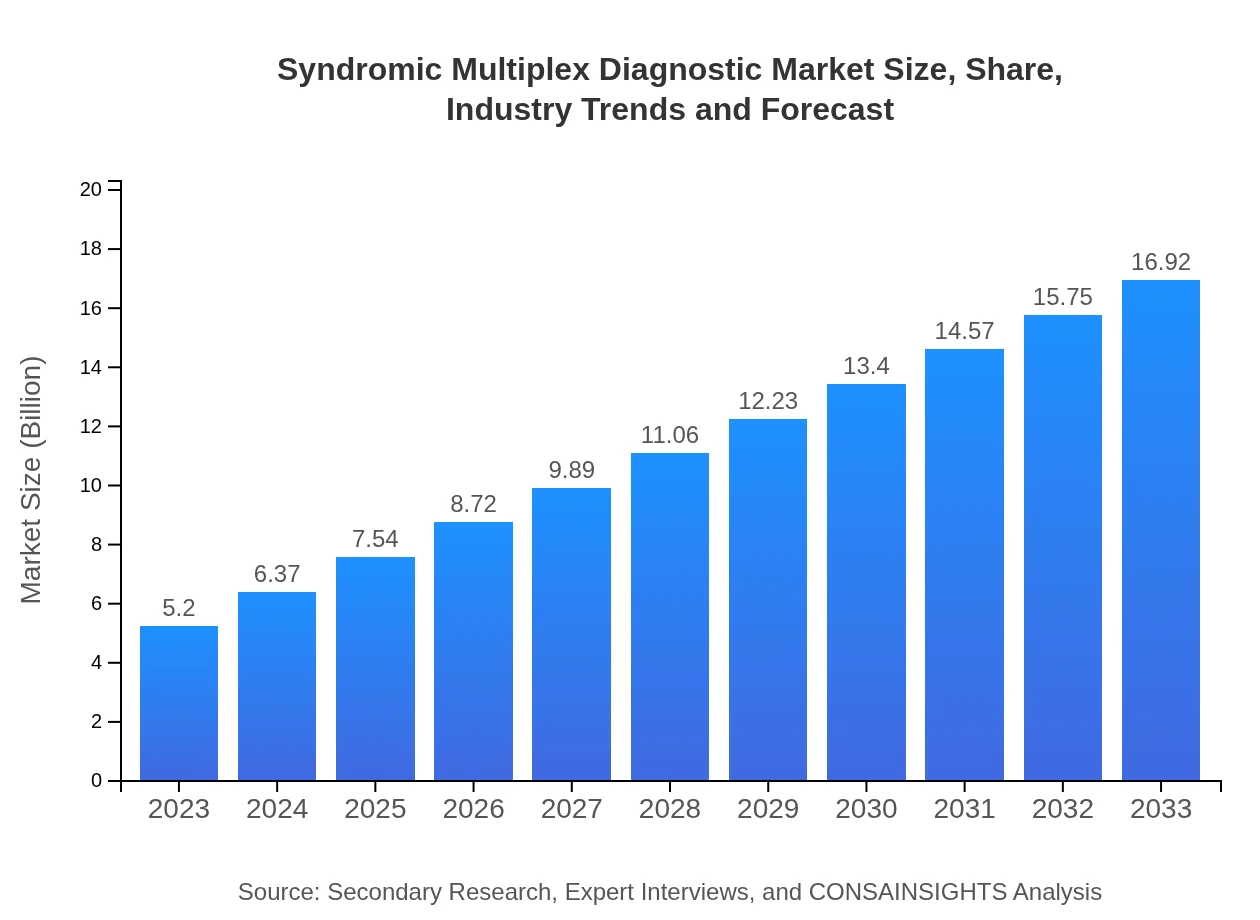
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 12% |
| 2033 Market Size | $16.92 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Syndromic Multiplex Diagnostic Market Overview
Customize Syndromic Multiplex Diagnostic Market Report market research report
- ✔ Get in-depth analysis of Syndromic Multiplex Diagnostic market size, growth, and forecasts.
- ✔ Understand Syndromic Multiplex Diagnostic's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Syndromic Multiplex Diagnostic
What is the Market Size & CAGR of Syndromic Multiplex Diagnostic market in 2023 and 2033?
Syndromic Multiplex Diagnostic Industry Analysis
Syndromic Multiplex Diagnostic Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Syndromic Multiplex Diagnostic Market Analysis Report by Region
Europe Syndromic Multiplex Diagnostic Market Report:
Europe's market is anticipated to grow from $1.30 billion in 2023 to $4.21 billion by 2033. Factors such as regulatory support for rapid diagnostics, increasing prevalence of infectious diseases, and rising investments in healthcare infrastructure are driving this growth.Asia Pacific Syndromic Multiplex Diagnostic Market Report:
The Asia Pacific region is experiencing significant growth in the Syndromic Multiplex Diagnostic market, projected to rise from $1.09 billion in 2023 to $3.56 billion by 2033. Factors include increasing healthcare expenditure, rising awareness of infectious diseases, and a growing number of diagnostic laboratories.North America Syndromic Multiplex Diagnostic Market Report:
North America represents the largest market for Syndromic Multiplex Diagnostics, forecasting an increase from $1.99 billion in 2023 to $6.49 billion by 2033. Strong dominance is attributed to high healthcare expenditure, technological advancements, and well-established healthcare systems supporting rapid diagnostic capabilities.South America Syndromic Multiplex Diagnostic Market Report:
In South America, the market is expected to grow from $0.16 billion in 2023 to $0.51 billion by 2033. Challenging healthcare infrastructure and limited access to advanced diagnostic platforms hinder rapid growth, though government initiatives are working to improve healthcare access.Middle East & Africa Syndromic Multiplex Diagnostic Market Report:
The Middle East and Africa market is projected to expand from $0.66 billion in 2023 to $2.14 billion by 2033. The growth is largely driven by increasing awareness of healthcare technologies and improving medical infrastructure in several countries in the region.Tell us your focus area and get a customized research report.
Syndromic Multiplex Diagnostic Market Analysis By Product
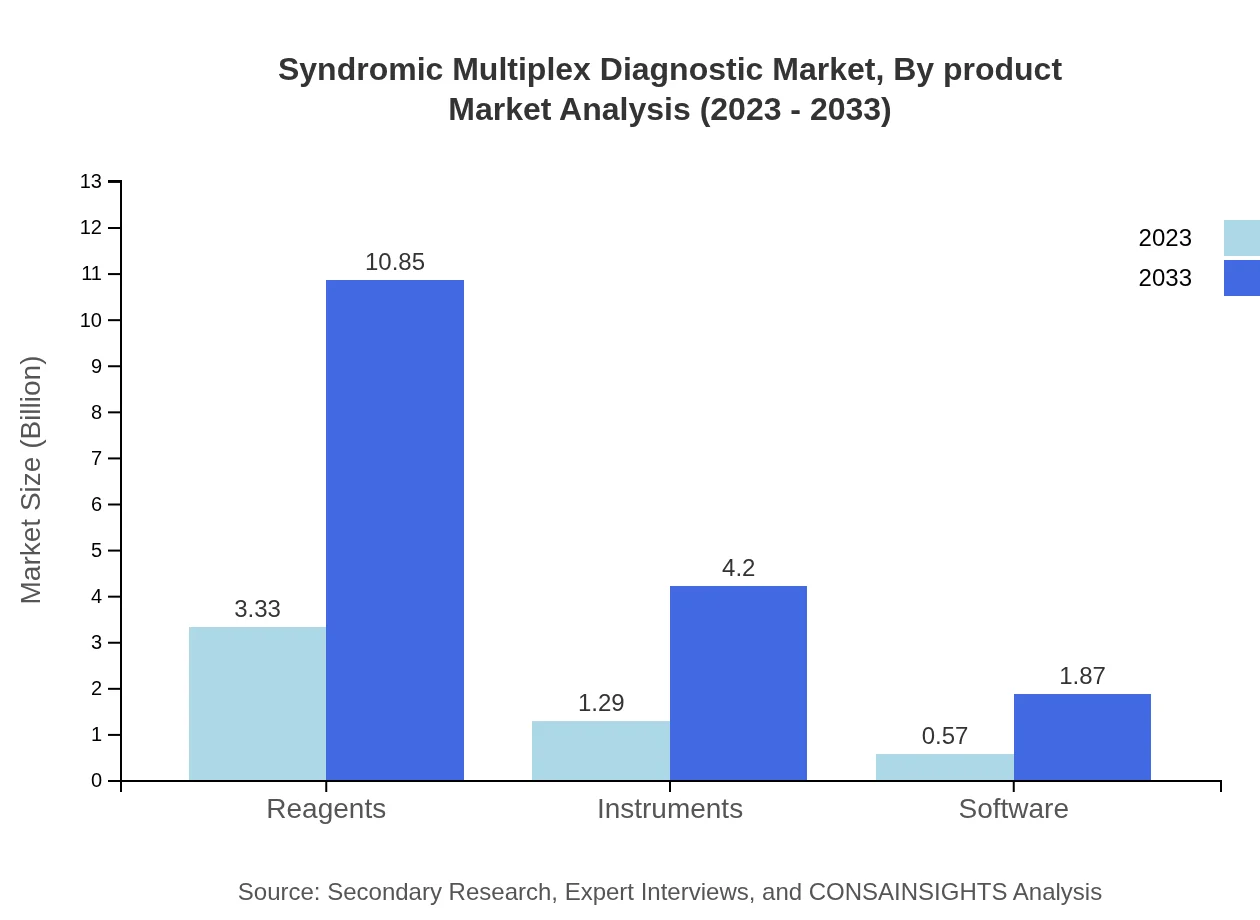
The product segment encompasses Reagents, Instruments, and Software. In 2023, the reagent market size is estimated at $3.33 billion, expanding to $10.85 billion by 2033, capturing 64.12% market share. Instruments are forecasted to grow from $1.29 billion to $4.20 billion, holding a 24.84% share, while software solutions will increase from $0.57 billion to $1.87 billion, representing an 11.04% share over the same period.
Syndromic Multiplex Diagnostic Market Analysis By Application
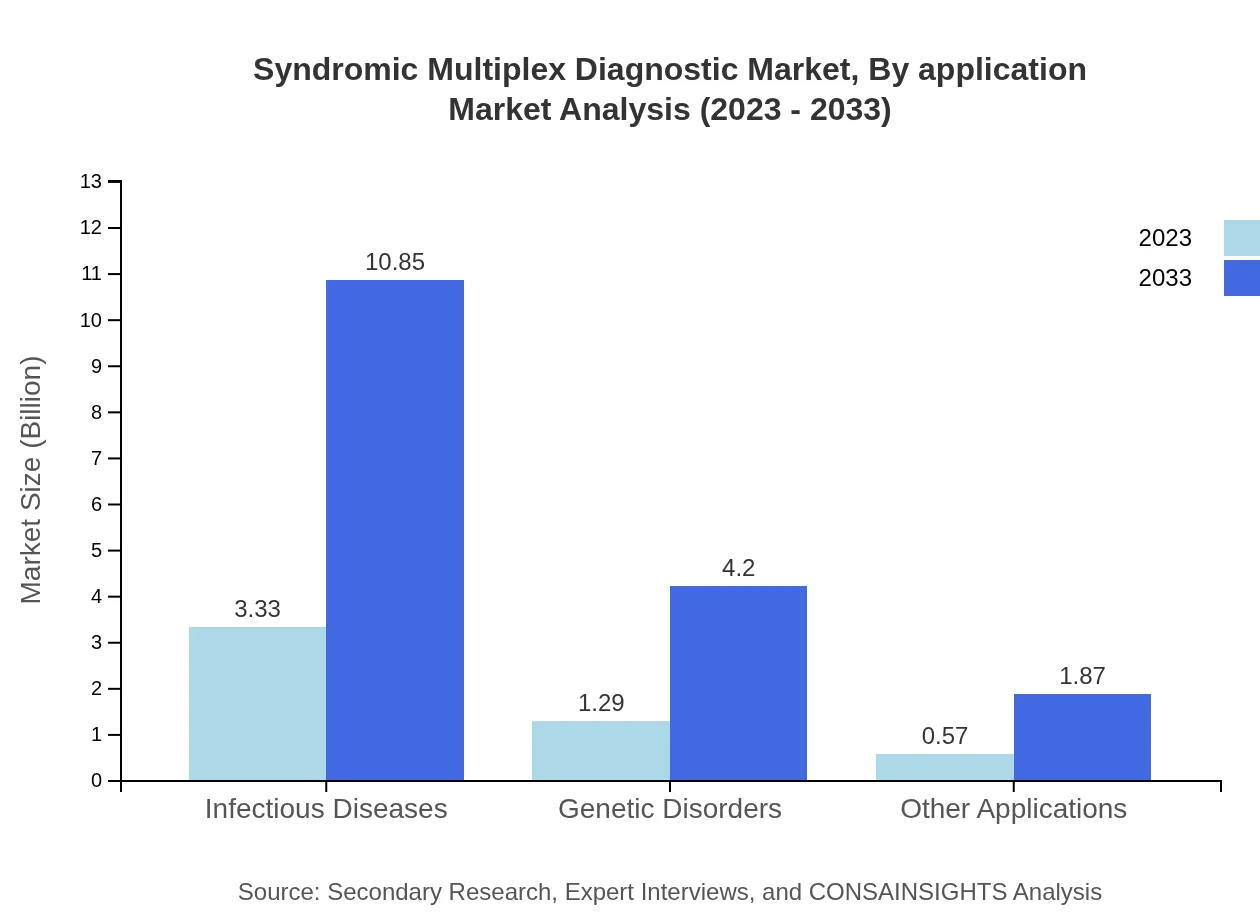
The application segment of the market includes Infectious Diseases, Genetic Disorders, and Other Applications. The Infectious Diseases segment is set to witness substantial growth, with its size projected to rise from $3.33 billion in 2023 to $10.85 billion by 2033, maintaining a 64.12% share. The Genetic Disorders application is anticipated to grow from $1.29 billion to $4.20 billion, holding a 24.84% market share, while other applications are predicted to increase from $0.57 billion to $1.87 billion, capturing 11.04% share.
Syndromic Multiplex Diagnostic Market Analysis By End User
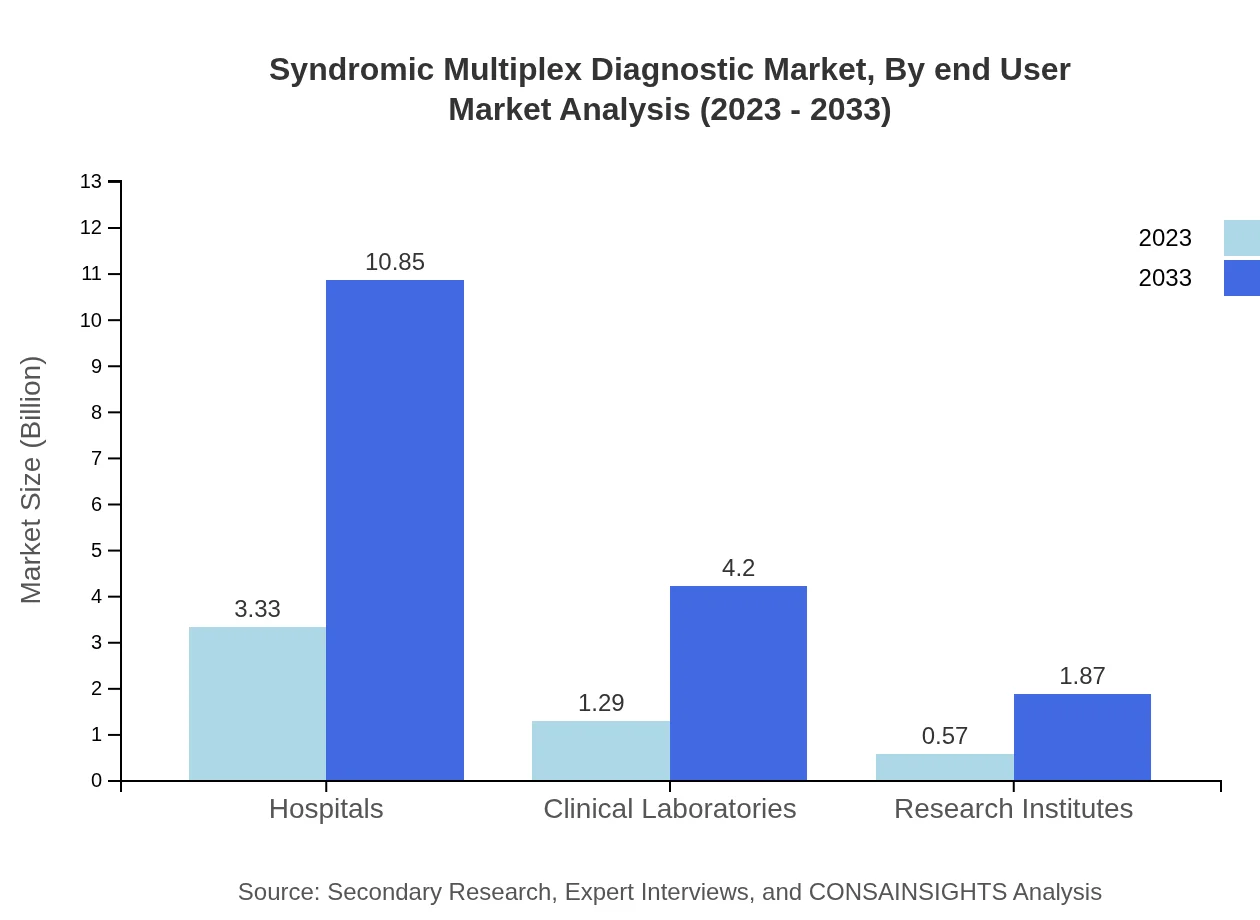
Hospitals, Clinical Laboratories, and Research Institutes define the end-user segment. Hospitals lead this category, with sizes expected to grow from $3.33 billion to $10.85 billion by 2033, maintaining a 64.12% share. Clinical Laboratories are projected to expand from $1.29 billion to $4.20 billion, making up 24.84% of the segment, while Research Institutes are estimated to rise from $0.57 billion to $1.87 billion, representing 11.04% share.
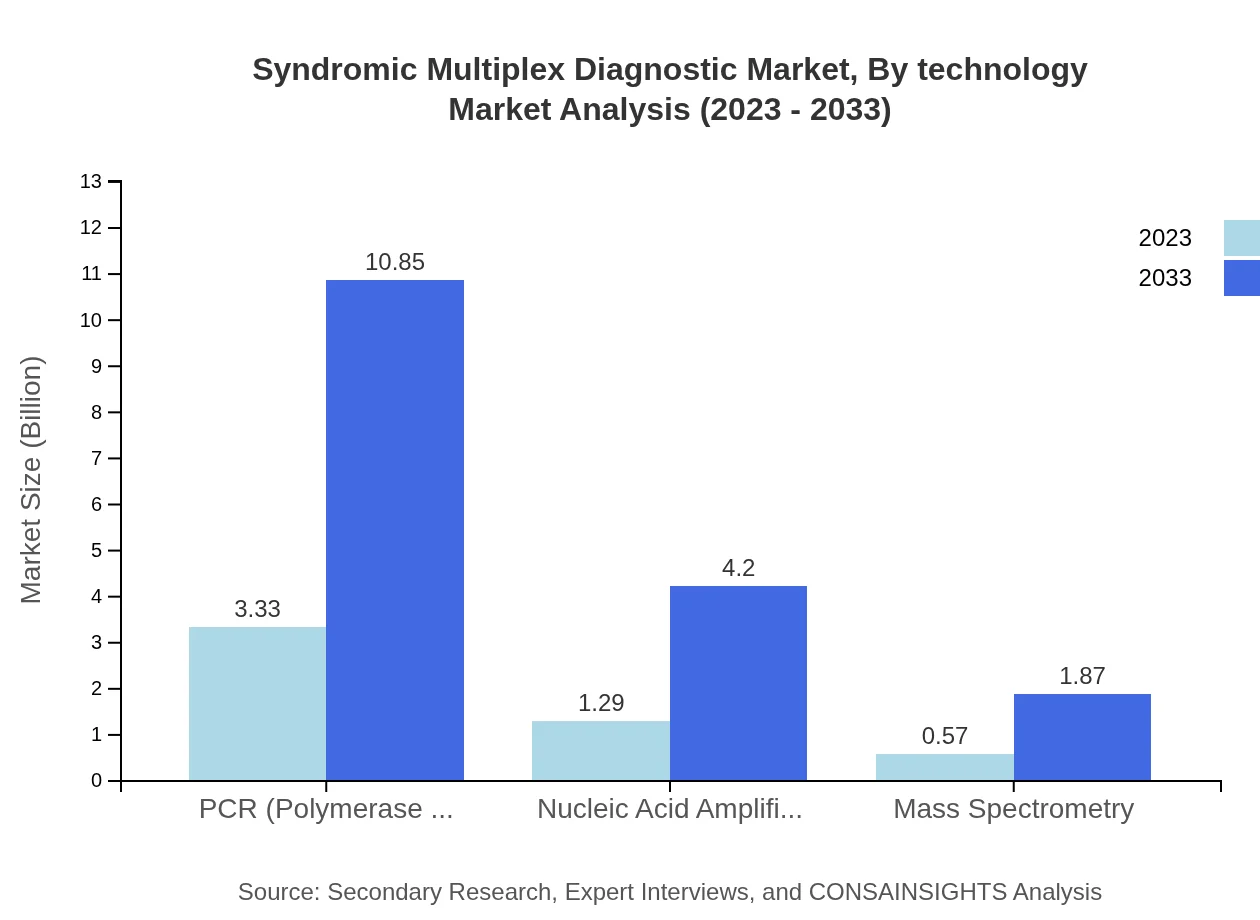
Syndromic Multiplex Diagnostic Market Analysis By Technology
The technology segment consists of PCR, Nucleic Acid Amplification, and Mass Spectrometry. PCR is the leading technology, estimated to grow from $3.33 billion in 2023 to $10.85 billion by 2033, holding 64.12% of the market share. Nucleic Acid Amplification will grow from $1.29 billion to $4.20 billion, capturing 24.84% share, while Mass Spectrometry is expected to increase from $0.57 billion to $1.87 billion, representing an 11.04% share.
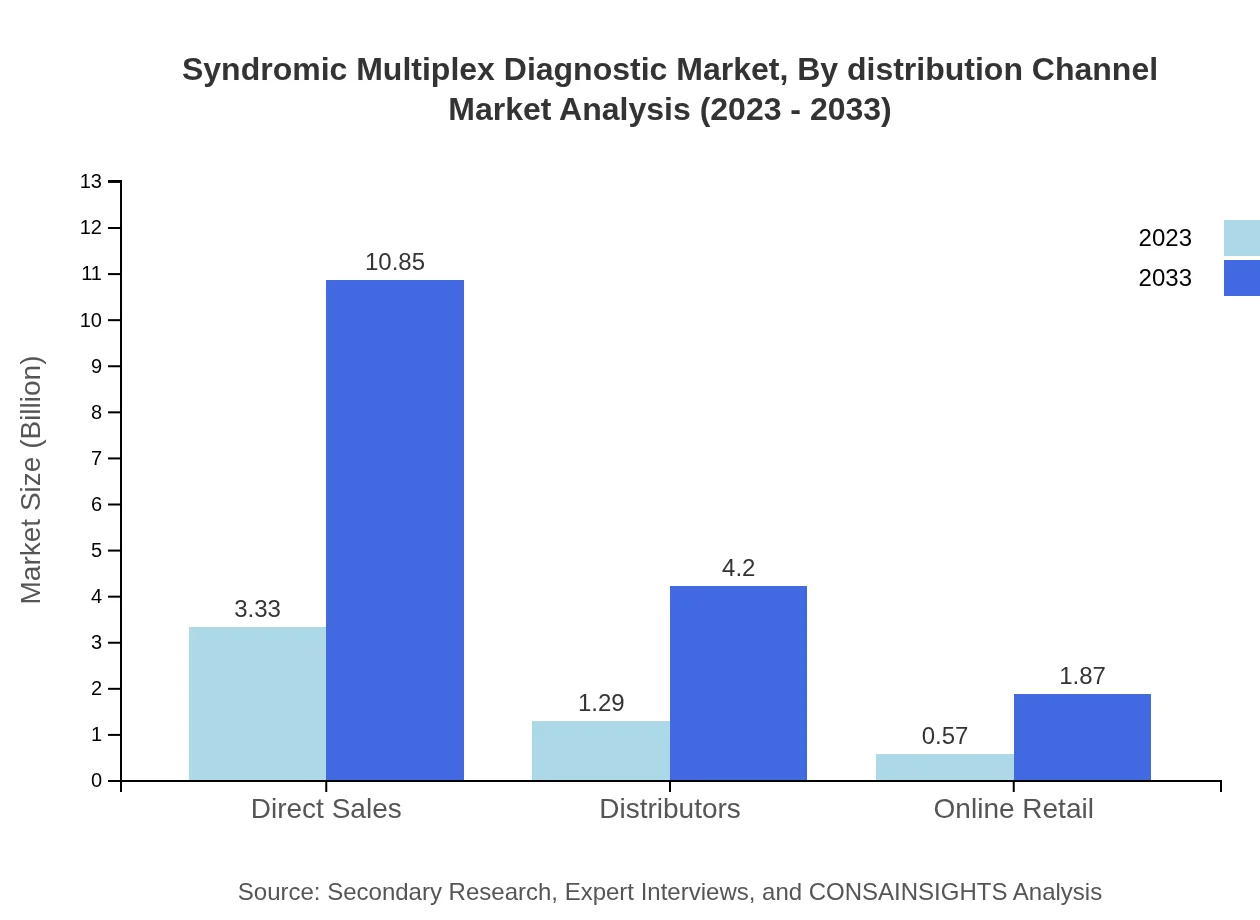
Syndromic Multiplex Diagnostic Market Analysis By Distribution Channel
The distribution channels include Direct Sales, Distributors, and Online Retail. Direct Sales is the predominant channel, with a market size from $3.33 billion in 2023 projected to reach $10.85 billion by 2033, holding a 64.12% share. Distributors are expected to grow from $1.29 billion to $4.20 billion, making up 24.84%, while Online Retail is anticipated to rise from $0.57 billion to $1.87 billion, capturing 11.04% share.
Syndromic Multiplex Diagnostic Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Syndromic Multiplex Diagnostic Industry
Roche Diagnostics:
Roche is a pioneer in the field of diagnostics, specializing in multiplex testing technologies that provide rapid and comprehensive testing solutions for infectious diseases.Abbott Laboratories:
Abbott is known for its innovative diagnostic solutions and has developed several multiplex diagnostic platforms that are widely used in laboratories and hospitals.Thermo Fisher Scientific:
Thermo Fisher is a global leader in serving science, providing technology and services for multiplex diagnostic testing across various clinical applications.Siemens Healthineers:
Siemens Healthineers focuses on strategic partnerships and advanced diagnostics, contributing significantly to the multiplex diagnostic market with its comprehensive product range.We're grateful to work with incredible clients.









FAQs
What is the market size of syndromic Multiplex Diagnostic?
The global syndromic multiplex diagnostic market was valued at $5.2 billion in 2023 and is projected to reach new heights by 2033, driven by a robust CAGR of 12%. This growth reflects the increasing demand for rapid and comprehensive diagnostic methods.
What are the key market players or companies in this syndromic Multiplex Diagnostic industry?
Key players in the syndromic multiplex diagnostic market include established diagnostic firms, biotechnology companies, and innovative startups focusing on multiplex testing technologies. These companies are pivotal in driving advancements and expanding the market significantly.
What are the primary factors driving the growth in the syndromic Multiplex Diagnostic industry?
Factors such as rising prevalence of infectious diseases, technological advancements in multiplex testing, and increased healthcare expenditure are propelling growth. Additionally, the demand for rapid and efficient diagnostics is critical for public health responses.
Which region is the fastest Growing in the syndromic Multiplex Diagnostic?
North America leads the syndromic multiplex diagnostic market with a valuation of $1.99 billion in 2023, growing to $6.49 billion by 2033. Europe and Asia Pacific also show substantial growth, reflecting a robust demand for diagnostic services.
Does ConsaInsights provide customized market report data for the syndromic Multiplex Diagnostic industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the syndromic multiplex diagnostic industry. Clients can receive in-depth, relevant insights to support strategic decision-making and market positioning.
What deliverables can I expect from this syndromic Multiplex Diagnostic market research project?
Deliverables typically include a comprehensive market report detailing market size, segmentation, growth trends, competitive landscape, and regional analyses. Additional insights into regulatory frameworks and market forecasts may also be available.
What are the market trends of syndromic Multiplex Diagnostic?
Key trends in the syndromic multiplex diagnostic market include increased adoption of PCR technology, emphasis on point-of-care testing, and innovation in diagnostic instruments. These trends are expected to shape the future landscape of healthcare diagnostics.