Tardive Dyskinesia Treatment Market Report
Published Date: 31 January 2026 | Report Code: tardive-dyskinesia-treatment
Tardive Dyskinesia Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report delivers an extensive analysis of the Tardive Dyskinesia Treatment market, examining trends, segmentation, regional insights, and market forecasts from 2023 to 2033. It provides key data and insights essential for stakeholders across the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
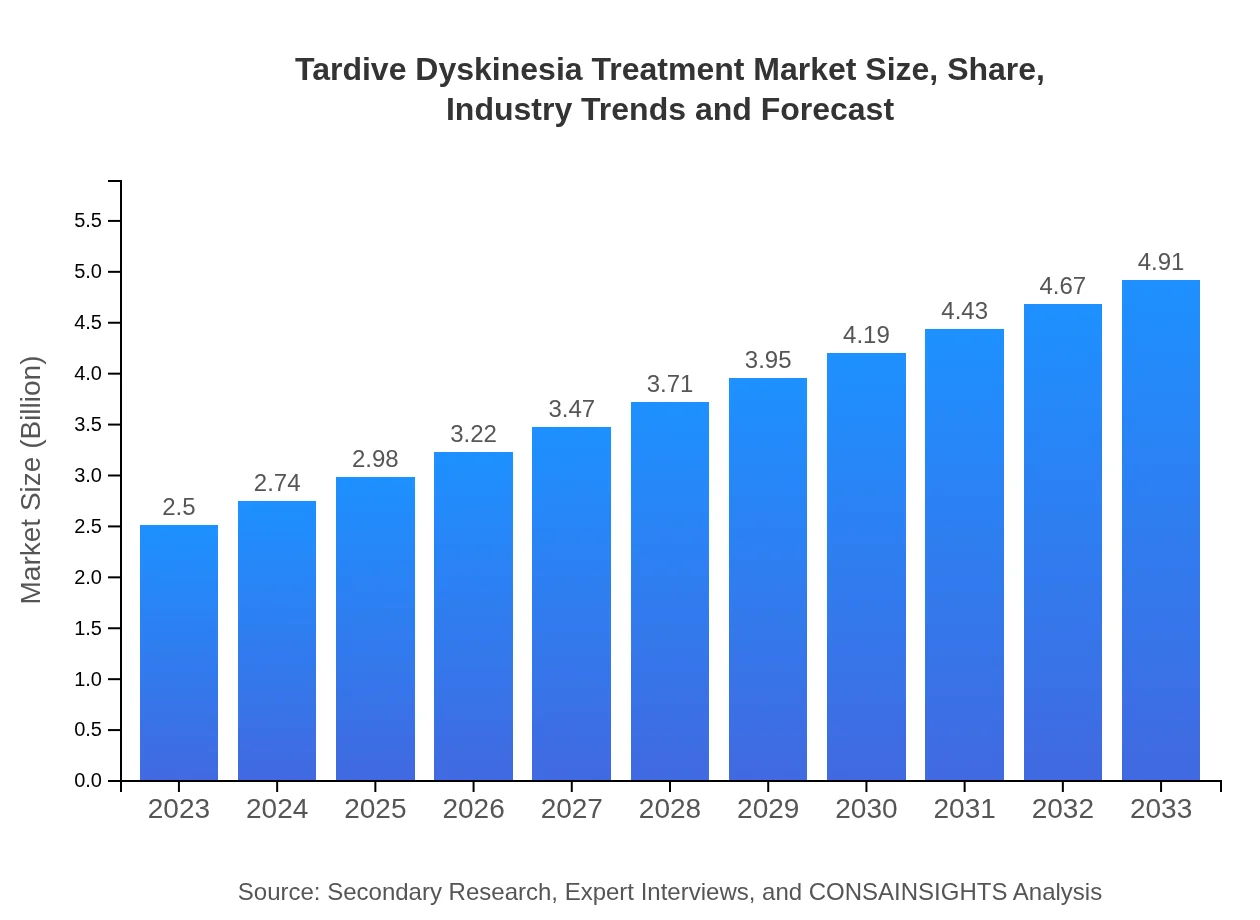
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Teva Pharmaceutical Industries Ltd., Neurocrine Biosciences, Inc., GlaxoSmithKline PLC, AbbVie Inc., Sunovion Pharmaceuticals Inc. |
| Last Modified Date | 31 January 2026 |
Tardive Dyskinesia Treatment Market Overview
Customize Tardive Dyskinesia Treatment Market Report market research report
- ✔ Get in-depth analysis of Tardive Dyskinesia Treatment market size, growth, and forecasts.
- ✔ Understand Tardive Dyskinesia Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Tardive Dyskinesia Treatment
What is the Market Size & CAGR of Tardive Dyskinesia Treatment market in 2023?
Tardive Dyskinesia Treatment Industry Analysis
Tardive Dyskinesia Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Tardive Dyskinesia Treatment Market Analysis Report by Region
Europe Tardive Dyskinesia Treatment Market Report:
Europe's Tardive Dyskinesia treatment market is expected to grow from $0.75 billion in 2023 to $1.48 billion by 2033. The region benefits from a robust regulatory framework supporting innovative therapies and an increasing awareness of TD among healthcare professionals, enabling enhanced diagnosis and treatment options.Asia Pacific Tardive Dyskinesia Treatment Market Report:
The Asia Pacific region shows substantial growth potential in the Tardive Dyskinesia treatment market, with a projected value of $0.54 billion in 2023, anticipated to rise to $1.06 billion by 2033. The increasing prevalence of mental health disorders and improved healthcare infrastructure contribute to this growth, alongside heightened government initiatives in mental health awareness and treatment.North America Tardive Dyskinesia Treatment Market Report:
North America stands as the largest market for Tardive Dyskinesia treatment, with a market size of $0.83 billion in 2023, projected to expand to $1.63 billion by 2033. Factors such as advanced healthcare systems, a high prevalence of TD due to widespread antipsychotic usage, and a focus on research and development drive growth in this region.South America Tardive Dyskinesia Treatment Market Report:
In South America, the Tardive Dyskinesia treatment market is currently valued at $0.05 billion, expecting to reach $0.09 billion by 2033. Economic barriers often limit treatment accessibility, but increased investment in healthcare facilities and mental health resources is gradually facilitating a better environment for treatment, ultimately benefiting patients suffering from TD.Middle East & Africa Tardive Dyskinesia Treatment Market Report:
The Middle East and Africa market is projected to grow from $0.33 billion to $0.65 billion in the same timeframe. The regional market growth is fueled by improving healthcare standards, increasing awareness regarding mental health disorders, and collaborations between government and private sectors to enhance treatment accessibility.Tell us your focus area and get a customized research report.
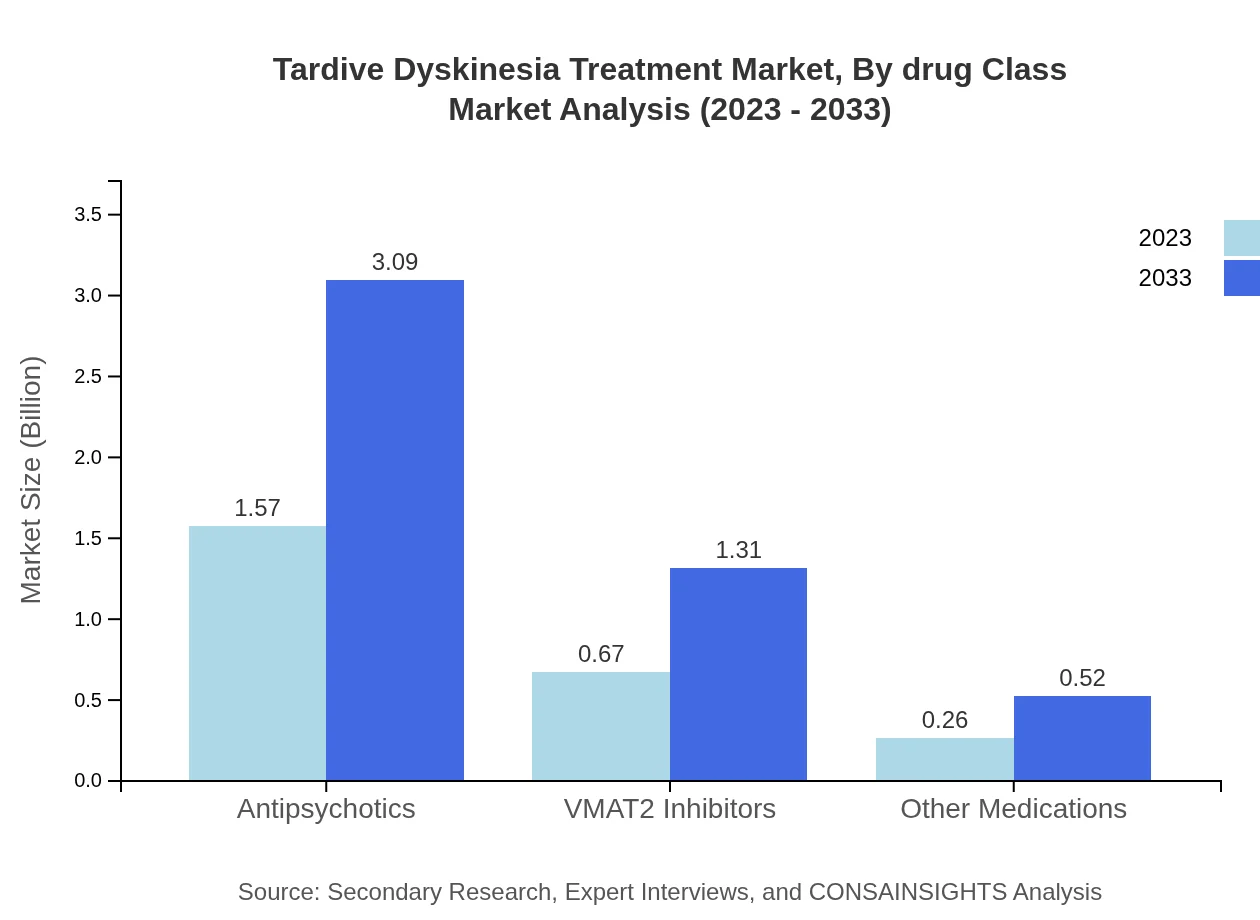
Tardive Dyskinesia Treatment Market Analysis By Drug Class
The Tardive Dyskinesia Treatment market can be segmented by drug class, comprising antipsychotics, VMAT2 inhibitors, and other medications. Antipsychotics represent the largest segment, expected to grow from $1.57 billion in 2023 to $3.09 billion by 2033, capturing approximately 62.79% of the market share. VMAT2 inhibitors follow, with market size forecasts reaching $1.31 billion by 2033.
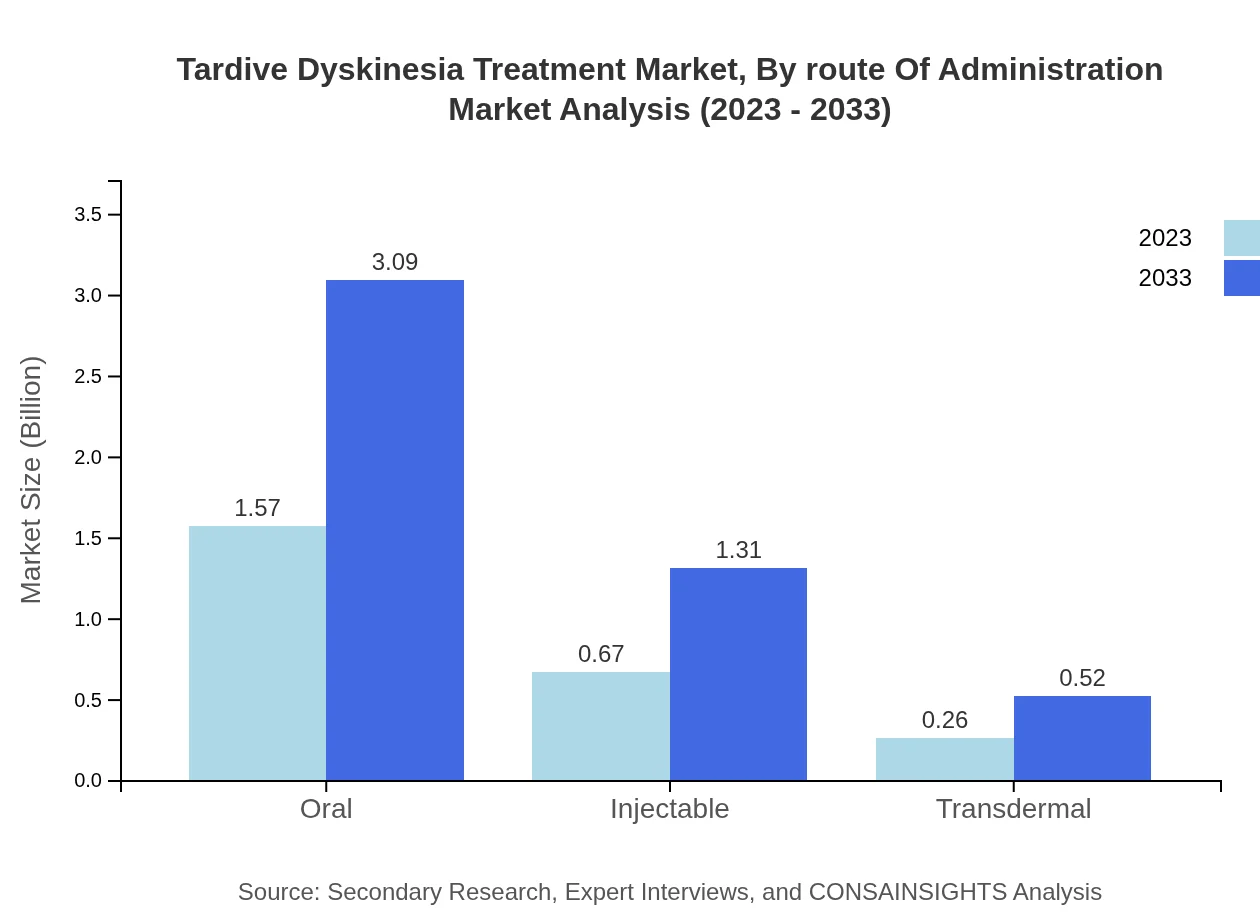
Tardive Dyskinesia Treatment Market Analysis By Route Of Administration
This market segment is classified into oral, injectable, and transdermal categories. Oral medications dominate the market, holding a share of approximately 62.79% in 2023, with projections of growth to $3.09 billion by 2033. Injectable administration methods are also witnessing a notable increase, primarily due to their efficacy in acute cases.
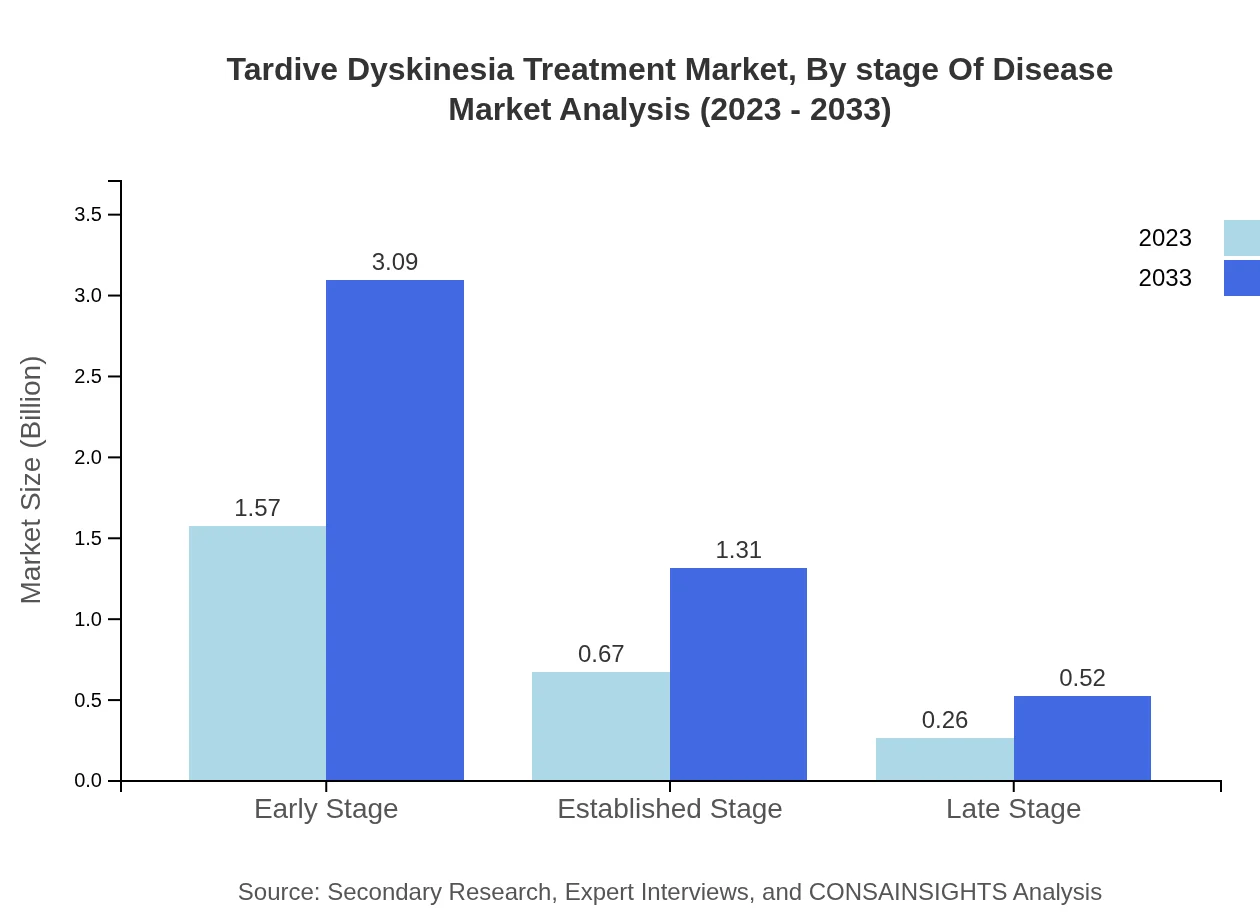
Tardive Dyskinesia Treatment Market Analysis By Stage Of Disease
The market segments concerning the stage of the disease include early, established, and late-stage TD treatments. Early-stage treatments are forecasted to encompass 62.79% of the market share, with a growth trajectory positioning its size at $3.09 billion by 2033, as early intervention approaches become more prevalent.
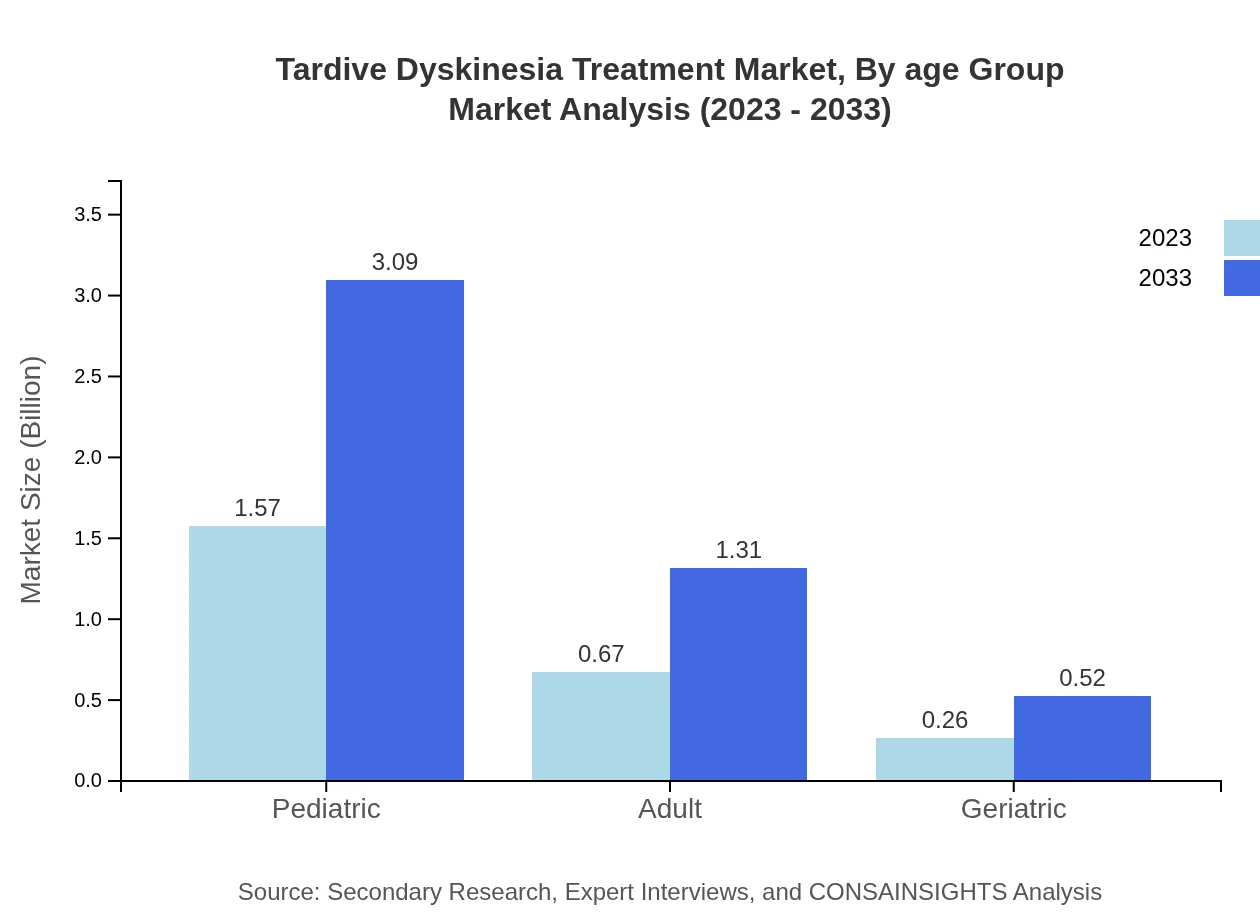
Tardive Dyskinesia Treatment Market Analysis By Age Group
Dividing the market by age group reveals significant share distribution: pediatric, adult, and geriatric segments. The pediatric population commands the highest share at 62.79%, projected to grow to $3.09 billion by 2033. This demonstrates an increasing recognition of the impact of TD in younger demographics.
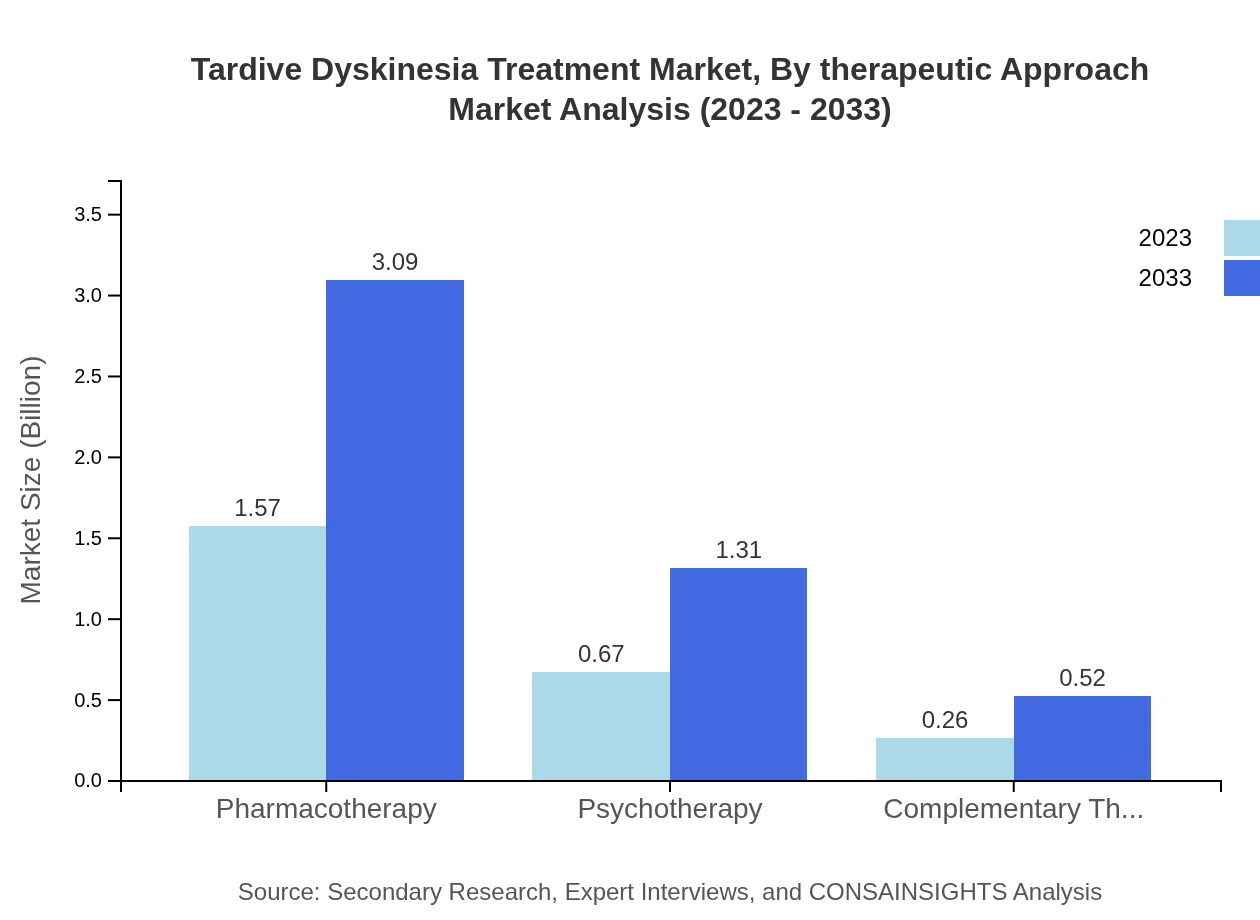
Tardive Dyskinesia Treatment Market Analysis By Therapeutic Approach
The market can also be categorized based on therapeutic approaches, including pharmacotherapy, psychotherapy, and complementary therapies. Pharmacotherapy remains the leading approach, holding a dominant share of 62.79% as of 2023 and expected growth to $3.09 billion by 2033, reflecting a high reliance on medication to manage TD symptoms.
Tardive Dyskinesia Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Tardive Dyskinesia Treatment Industry
Teva Pharmaceutical Industries Ltd.:
Teva is a global leader in generic and specialty pharmaceuticals, particularly known for its innovative treatments for neurological disorders, including TD.Neurocrine Biosciences, Inc.:
Neurocrine focuses on developing treatments for neurological and endocrine diseases, particularly involving VMAT2 inhibitors, which are crucial for TD management.GlaxoSmithKline PLC:
GSK is renowned for its vast portfolio of pharmaceuticals including antipsychotic medications essential for TD treatment.AbbVie Inc.:
AbbVie is key in the development of specialized treatment options and continues to invest heavily in research to improve TD therapies.Sunovion Pharmaceuticals Inc.:
Sunovion specializes in developing innovative medicines to treat and improve the lives of individuals with serious mental illnesses, including TD.We're grateful to work with incredible clients.









FAQs
What is the market size of tardive Dyskinesia Treatment?
The tardive dyskinesia treatment market is estimated to be worth approximately $2.5 billion in 2023, with a projected CAGR of 6.8% over the next decade. This growth reflects increasing awareness and advancements in therapies, driven by rising patient populations.
What are the key market players or companies in this tardive Dyskinesia Treatment industry?
Key players in the tardive dyskinesia treatment market include pharmaceutical giants that focus on neurology and mental health. These companies are pivotal in research and innovation and are continuously exploring new therapies to improve patient outcomes in managing this condition.
What are the primary factors driving the growth in the tardive Dyskinesia treatment industry?
Several factors are fueling growth in the tardive dyskinesia treatment industry, including the rising incidence of psychiatric conditions that require antipsychotic medications, increasing patient awareness about the side effects, and advancements in drug formulations improving therapy effectiveness.
Which region is the fastest Growing in the tardive Dyskinesia treatment market?
The fastest-growing region in the tardive dyskinesia treatment market is projected to be North America, where market size is expected to grow from $0.83 billion in 2023 to $1.63 billion by 2033, driven by a strong healthcare infrastructure and increased research funding.
Does Consainsights provide customized market report data for the tardive Dyskinesia treatment industry?
Yes, Consainsights offers customized market reports tailored to the specific needs of clients in the tardive dyskinesia treatment industry. These reports can encompass various aspects such as market trends, competitor analysis, and potential growth areas.
What deliverables can I expect from this tardive Dyskinesia treatment market research project?
Deliverables from a tardive dyskinesia treatment market research project include comprehensive market analysis reports, growth forecasts, trends analysis, competitive landscape evaluations, and customized insights based on client specifications, ensuring informed decision-making.
What are the market trends of tardive Dyskinesia treatment?
Current market trends in tardive dyskinesia treatment include a shift towards novel therapeutic agents, personalized medicine approaches, increased focus on patient-centered care, and integration of technology in treatment protocols, enhancing overall management and patient outcomes.