Therapeutic Drug Monitoring Market Report
Published Date: 31 January 2026 | Report Code: therapeutic-drug-monitoring
Therapeutic Drug Monitoring Market Size, Share, Industry Trends and Forecast to 2033
This market report provides a comprehensive analysis of the Therapeutic Drug Monitoring (TDM) market from 2023 to 2033, highlighting market trends, size forecasts, and regional insights to facilitate strategic planning and decision-making for stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
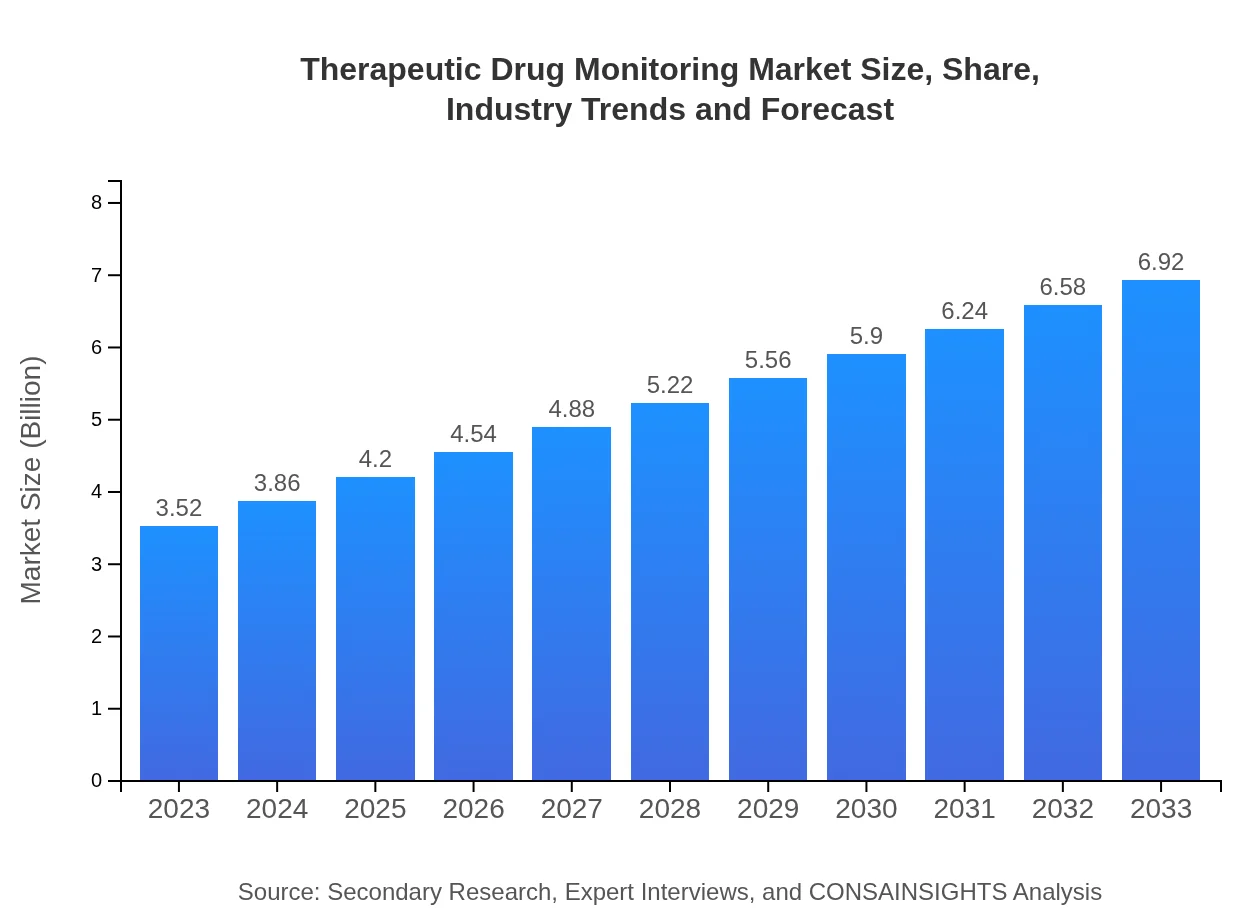
| 2023 Market Size | $3.52 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $6.92 Billion |
| Top Companies | Roche Diagnostics, Thermo Fisher Scientific, Siemens Healthineers, Abbott Laboratories |
| Last Modified Date | 31 January 2026 |
Therapeutic Drug Monitoring Market Overview
Customize Therapeutic Drug Monitoring Market Report market research report
- ✔ Get in-depth analysis of Therapeutic Drug Monitoring market size, growth, and forecasts.
- ✔ Understand Therapeutic Drug Monitoring's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Therapeutic Drug Monitoring
What is the Market Size & CAGR of Therapeutic Drug Monitoring market in 2033?
Therapeutic Drug Monitoring Industry Analysis
Therapeutic Drug Monitoring Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Therapeutic Drug Monitoring Market Analysis Report by Region
Europe Therapeutic Drug Monitoring Market Report:
Europe's TDM market is expected to grow from $0.91 billion in 2023 to $1.79 billion by 2033. The region benefits from well-established healthcare systems and increasing initiatives aimed at enhancing patient safety through effective medication management. Furthermore, rising investments in precision medicine are propelling TDM practices.Asia Pacific Therapeutic Drug Monitoring Market Report:
In the Asia Pacific region, the TDM market size reached $0.72 billion in 2023, projected to grow to $1.41 billion by 2033. This growth is driven by increasing healthcare investments, rising chronic disease prevalence, and a growing focus on personalized medicine. Countries like China and India are expected to lead this growth due to their expanding healthcare infrastructure and growing pharmaceutical markets.North America Therapeutic Drug Monitoring Market Report:
North America, particularly the United States, represents the largest TDM market, valued at $1.24 billion in 2023 and anticipated to reach $2.43 billion by 2033. Key drivers include high healthcare expenditure, the widespread adoption of advanced monitoring technologies, and a strong emphasis on research and development in pharmacogenomics.South America Therapeutic Drug Monitoring Market Report:
South America's TDM market size was valued at $0.31 billion in 2023, with expectations to reach $0.61 billion by 2033. The market is largely influenced by the rising demand for cost-effective monitoring solutions in clinical settings and improving regulatory frameworks that support advanced diagnostic capabilities.Middle East & Africa Therapeutic Drug Monitoring Market Report:
The Middle East and Africa region has a TDM market size of $0.35 billion in 2023, projected to grow to $0.68 billion by 2033. Market growth is supported by improving healthcare access, increased disease awareness, and growing investments in healthcare technologies.Tell us your focus area and get a customized research report.
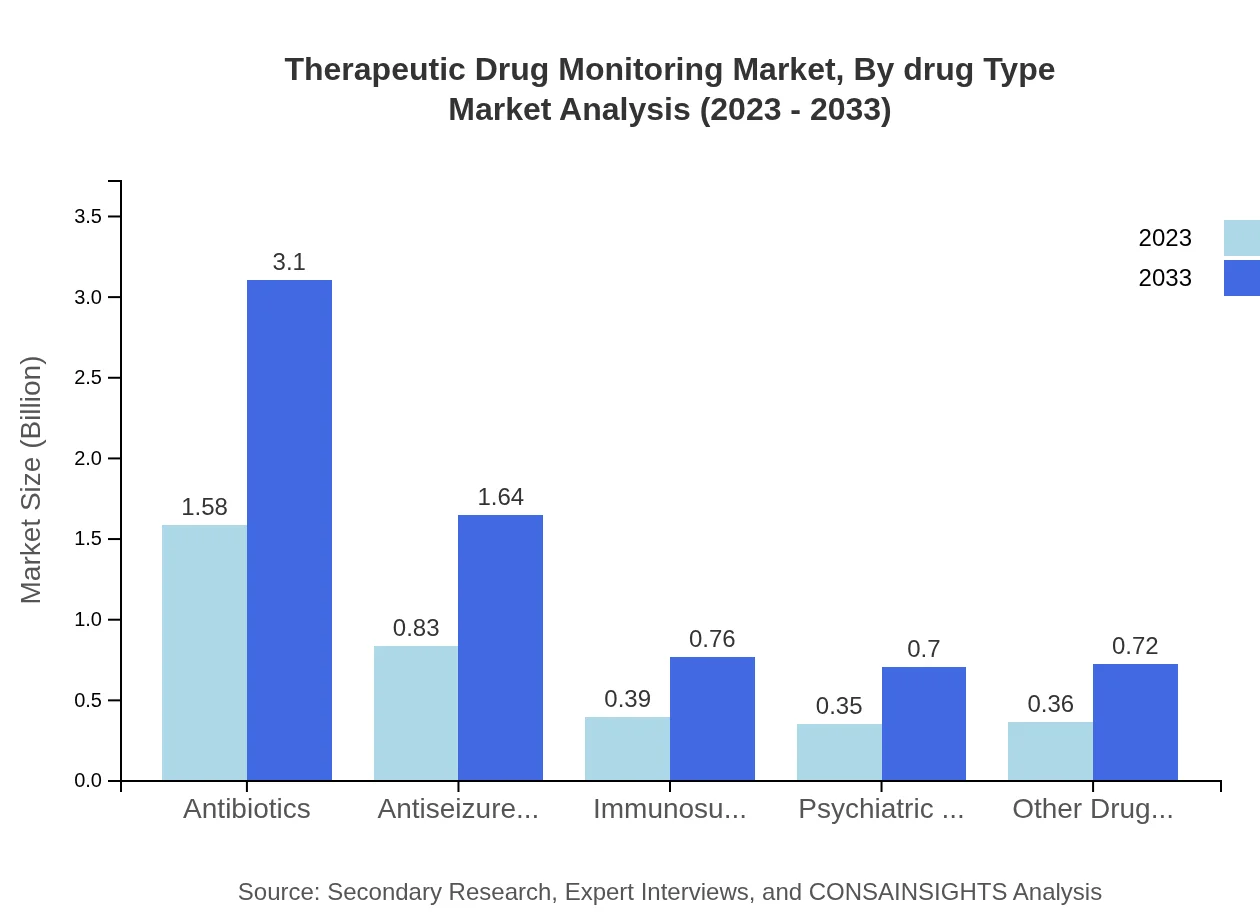
Therapeutic Drug Monitoring Market Analysis By Drug Type
The Therapeutic Drug Monitoring market by drug type includes various categories significantly impacting the market. Antibiotics dominate the segment with a market size of $1.58 billion in 2023, expected to reach $3.10 billion by 2033. Antiseizure medications follow with an expected growth from $0.83 billion to $1.64 billion. The growing number of patients requiring ongoing monitoring of these drugs is fueling this segment's expansion. Other significant drug types include immunosuppressants, psychiatric medications, and antiseizure medications.
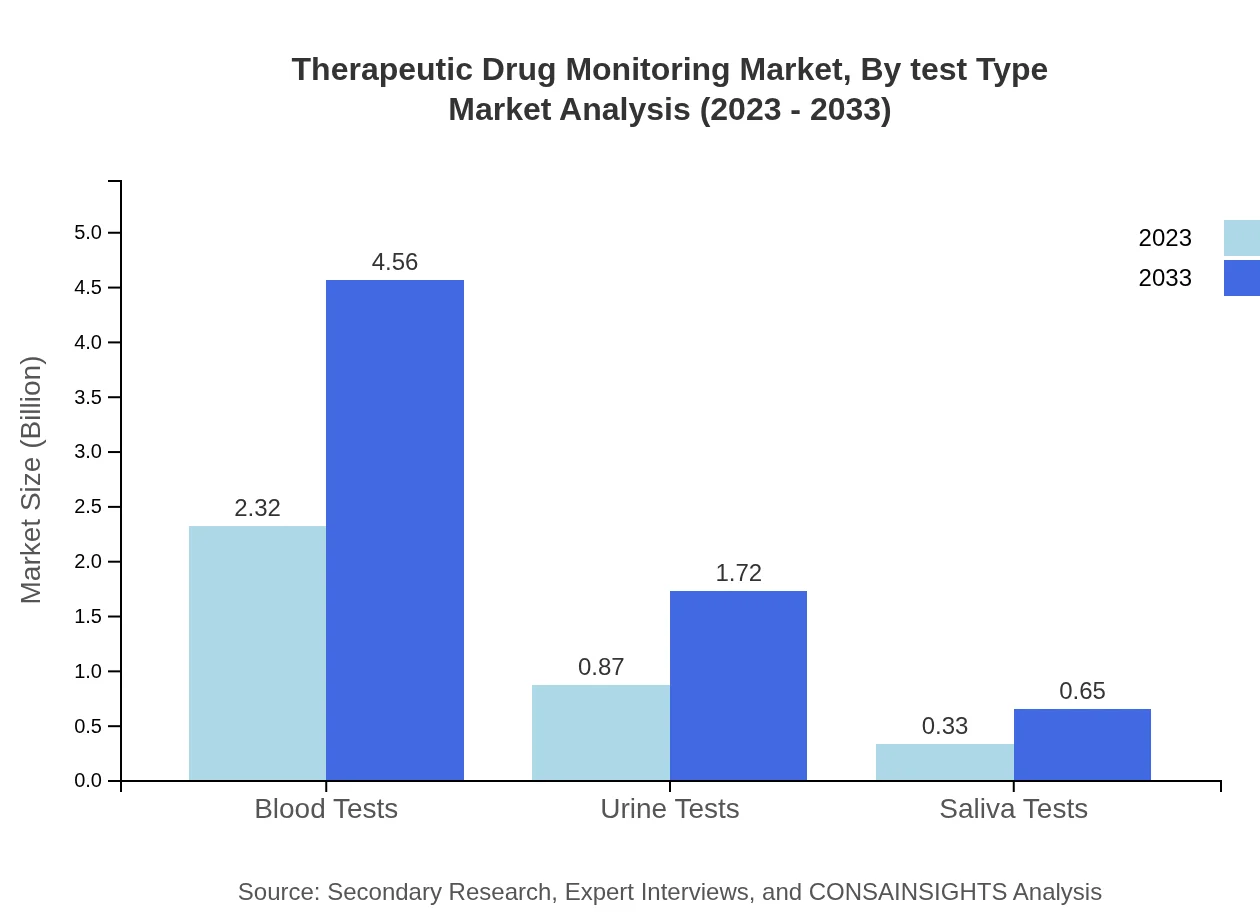
Therapeutic Drug Monitoring Market Analysis By Test Type
Test types in the TDM market include blood tests, urine tests, and saliva tests. Blood tests lead the market, with a size of $2.32 billion in 2023, projected to increase to $4.56 billion by 2033. Urine tests and saliva tests, although smaller, are also significant, offering alternative monitoring methods in suitable clinical contexts.
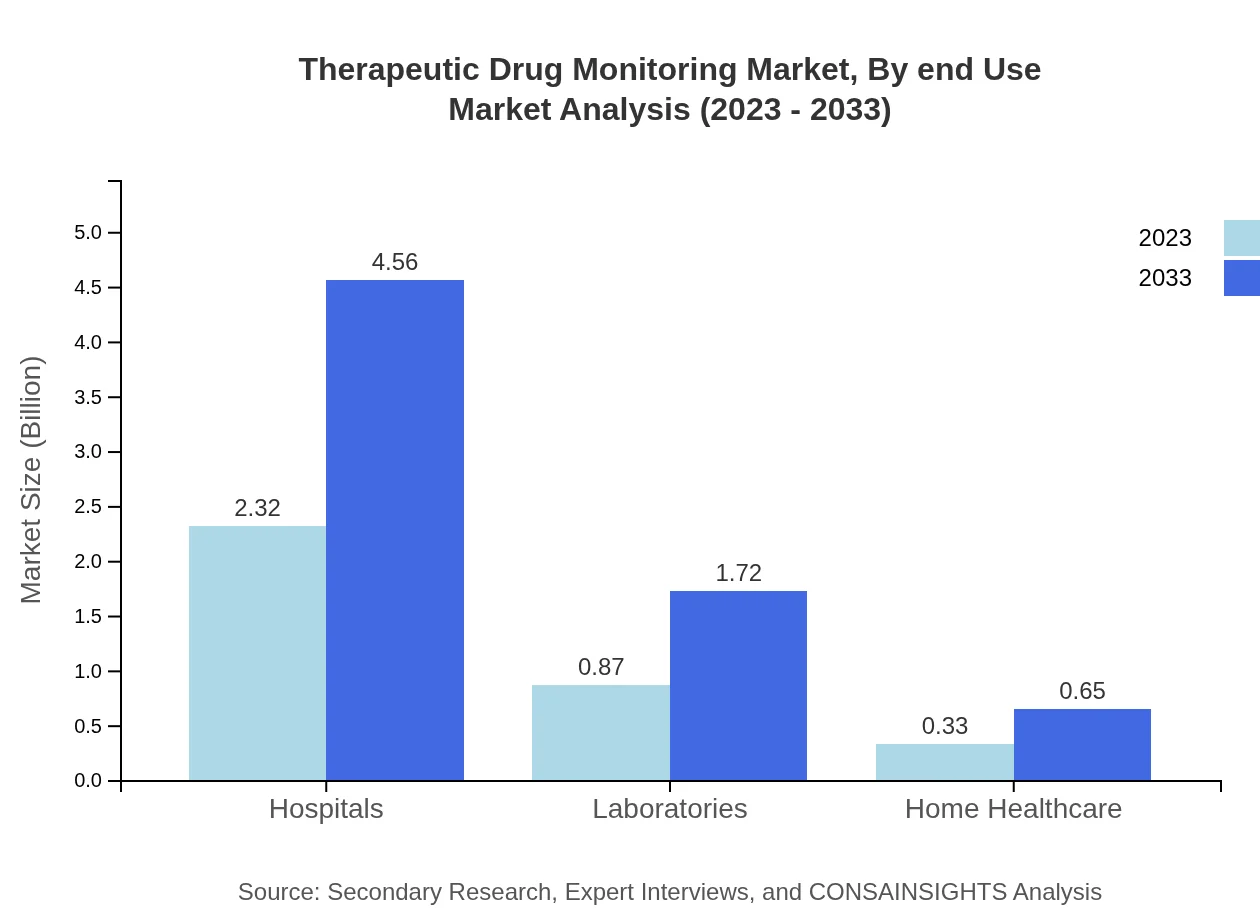
Therapeutic Drug Monitoring Market Analysis By End Use
End-user segmentation highlights hospitals as the primary users of TDM services, with a market size of $2.32 billion in 2023 expected to grow to $4.56 billion by 2033. Laboratories also play a critical role, contributing a market size of $0.87 billion with a growth projection to $1.72 billion. Home healthcare settings are emerging as an essential segment due to the shift towards patient-centered care.
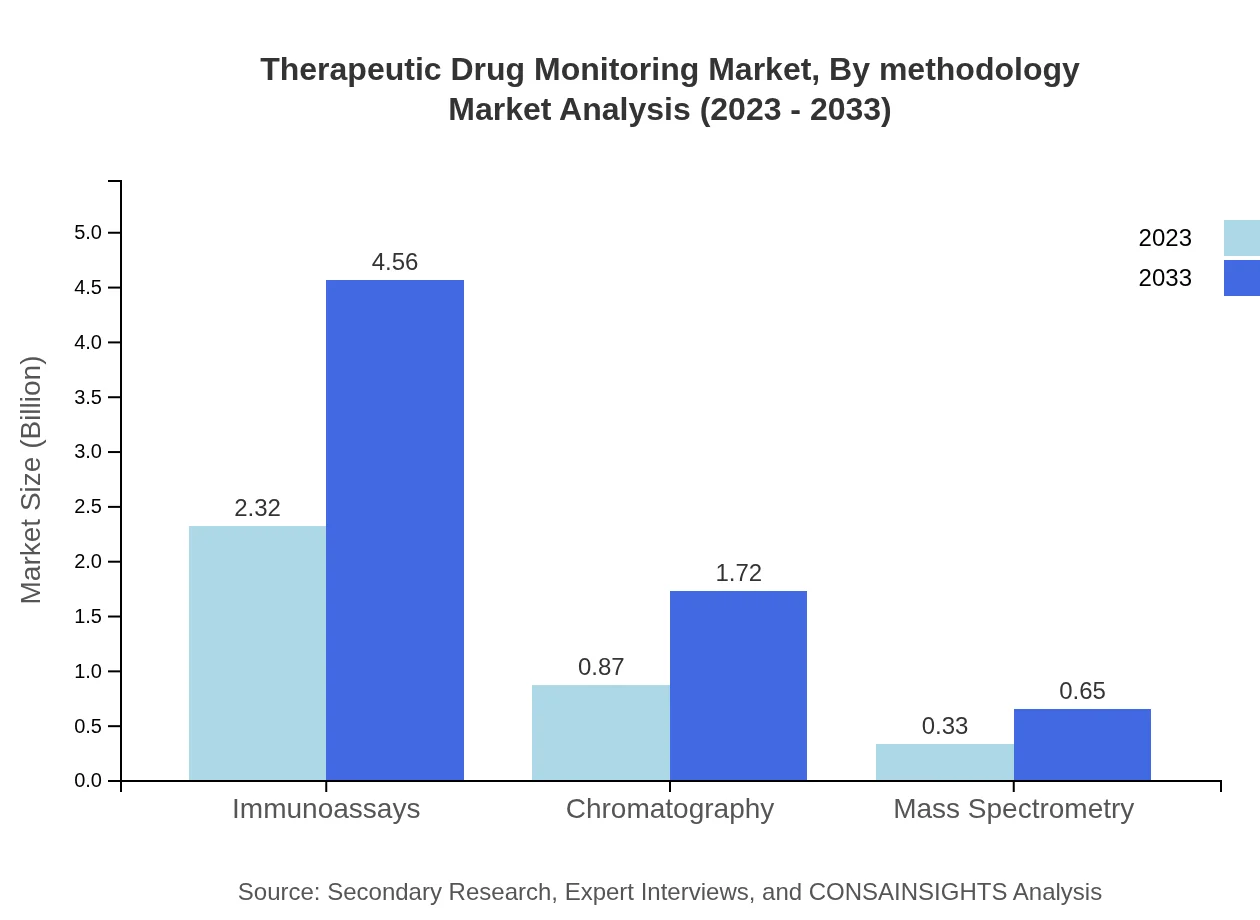
Therapeutic Drug Monitoring Market Analysis By Methodology
Methodology segmentation includes techniques like immunoassays, chromatography, and mass spectrometry. Immunoassays are the predominant method, representing a market size of $2.32 billion, while chromatography and mass spectrometry follow. The advancement in these technologies is elevating monitoring precision and efficiency.
Therapeutic Drug Monitoring Market Analysis By Region Specific Applications
Region-specific applications account for the TDM market variation across different markets. North America remains the strongest market due to high awareness and healthcare spending, while Asia Pacific shows rapid growth potential driven by technological adoption and modernized healthcare infrastructures.
Therapeutic Drug Monitoring Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Therapeutic Drug Monitoring Industry
Roche Diagnostics:
A leading player known for its innovative diagnostic solutions, Roche Diagnostics significantly contributes to the TDM market with advanced products and services tailored to medication monitoring.Thermo Fisher Scientific:
Thermo Fisher Scientific specializes in laboratory instrumentation and reagents, offering TDM tools that enhance testing accuracy and reliability for healthcare providers.Siemens Healthineers:
As a prominent name in medical technology, Siemens Healthineers provides diagnostic solutions that foster efficient therapeutic drug monitoring, ensuring optimal patient outcomes.Abbott Laboratories:
Abbott is renowned for its diagnostic devices and technologies, playing a crucial role in advancing TDM practices across various therapeutic areas.We're grateful to work with incredible clients.









FAQs
What is the market size of therapeutic Drug Monitoring?
The therapeutic drug monitoring (TDM) market is projected to reach approximately $3.52 billion by 2033, growing at a CAGR of 6.8% from its current size. This growth reflects the increasing demand for precise medication management to optimize patient outcomes.
What are the key market players or companies in this therapeutic Drug Monitoring industry?
Key players in the therapeutic drug monitoring market include pharmaceutical giants, diagnostic laboratories, and technology firms specializing in medical devices and software for drug testing. Their innovative approaches and partnerships are shaping market dynamics.
What are the primary factors driving the growth in the therapeutic drug monitoring industry?
Factors driving growth in the TDM market include the rising prevalence of chronic diseases, advancements in laboratory technology, and growing adoption of personalized medicine. Increased awareness among clinicians about the benefits of TDM further stimulates market expansion.
Which region is the fastest Growing in the therapeutic drug monitoring?
The fastest-growing region in the therapeutic drug monitoring market is North America, expected to grow from $1.24 billion in 2023 to $2.43 billion by 2033. This growth is fueled by advanced healthcare infrastructure and increasing demand for personalized therapies.
Does ConsaInsights provide customized market report data for the therapeutic drug monitoring industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the therapeutic drug monitoring industry. This includes in-depth analyses, market forecasts, and insights designed to support strategic decision-making.
What deliverables can I expect from this therapeutic drug monitoring market research project?
From the therapeutic drug monitoring market research project, clients can expect comprehensive reports detailing market size, segmentation data, regional analyses, key trends, and competitive landscapes, providing valuable insights to navigate the market effectively.
What are the market trends of therapeutic drug monitoring?
Current trends in the therapeutic drug monitoring market include increased utilization of home healthcare solutions, advancements in testing technologies, and a shift towards more personalized medicine, enhancing patient outcomes across various therapeutic areas.