Therapeutic Stents Market Report
Published Date: 31 January 2026 | Report Code: therapeutic-stents
Therapeutic Stents Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the therapeutic stents market, covering market size, trends, segmentation, and forecasts until 2033. Extensive insights are offered on regional performances and the leading companies in the market, facilitating informed decision-making for stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
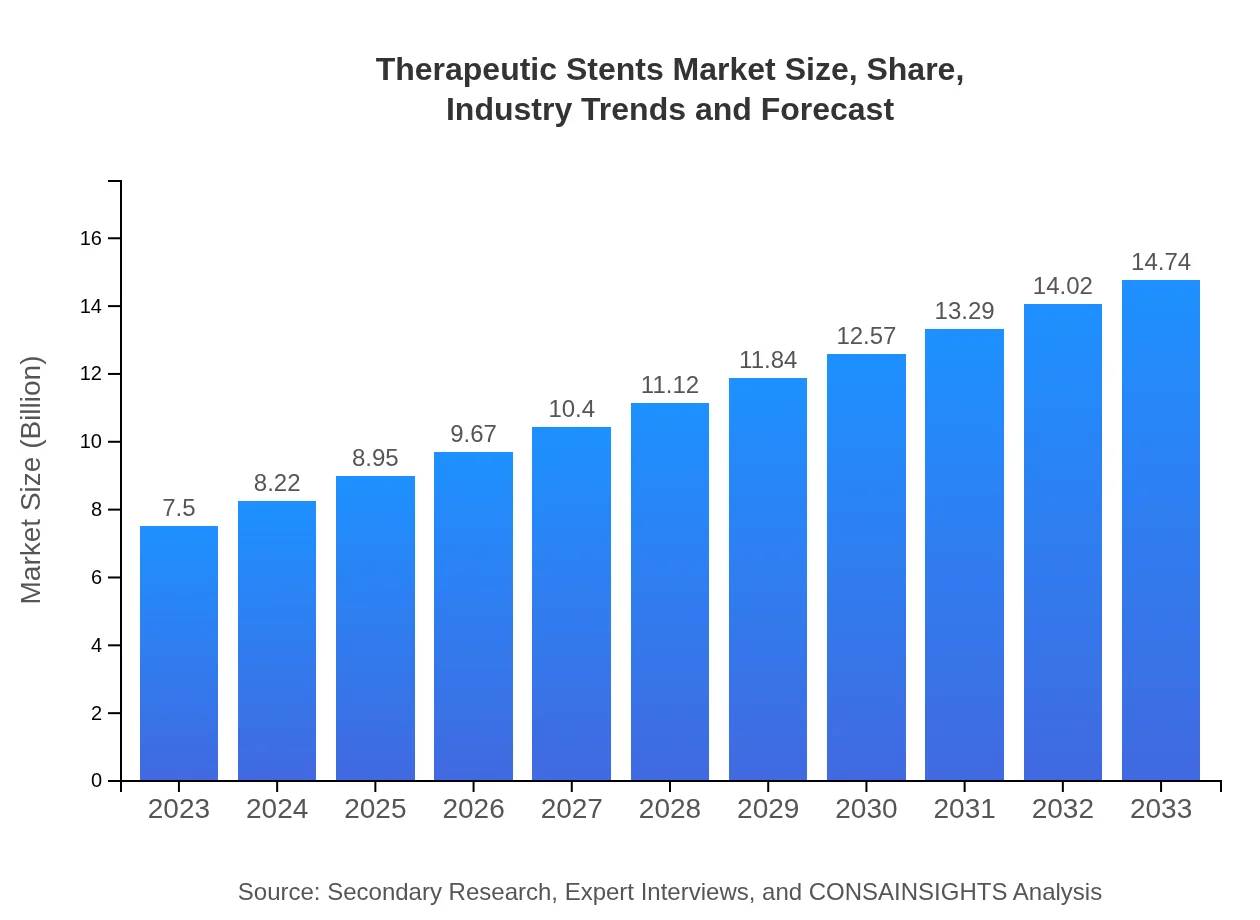
| 2023 Market Size | $7.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $14.74 Billion |
| Top Companies | Abbott Laboratories, Boston Scientific Corporation, Medtronic plc, Cordis (a Cardinal Health company), Terumo Corporation |
| Last Modified Date | 31 January 2026 |
Therapeutic Stents Market Overview
Customize Therapeutic Stents Market Report market research report
- ✔ Get in-depth analysis of Therapeutic Stents market size, growth, and forecasts.
- ✔ Understand Therapeutic Stents's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Therapeutic Stents
What is the Market Size & CAGR of Therapeutic Stents market in 2023?
Therapeutic Stents Industry Analysis
Therapeutic Stents Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Therapeutic Stents Market Analysis Report by Region
Europe Therapeutic Stents Market Report:
The European therapeutic stents market is estimated to grow from $2.01 billion in 2023 to $3.96 billion by 2033. Strong regulatory support for medical devices and a growing population suffering from cardiovascular diseases contribute significantly to market expansion in this region.Asia Pacific Therapeutic Stents Market Report:
The Asia Pacific therapeutic stents market is expected to grow from $1.53 billion in 2023 to $3.01 billion by 2033, indicating a strong growth trajectory driven by increasing healthcare expenditure and technological advancements in medical devices. Favorable government regulations further encourage the adoption of innovative stents within the region.North America Therapeutic Stents Market Report:
North America holds the largest share of the therapeutic stents market, valued at $2.76 billion in 2023 and expected to reach $5.43 billion by 2033. This growth is attributed to advanced healthcare infrastructure, high adoption rates of innovative stent technologies, and extensive research and development efforts by leading manufacturers.South America Therapeutic Stents Market Report:
In 2023, the South American therapeutic stents market is valued at $0.45 billion, with a projected growth to $0.88 billion by 2033. Factors such as rising awareness of cardiovascular diseases and healthcare accessibility drive market growth, although challenges related to high costs and regulatory hurdles persist.Middle East & Africa Therapeutic Stents Market Report:
The Middle East and Africa market is anticipated to increase from $0.75 billion in 2023 to $1.47 billion by 2033. The growing prevalence of heart disease and improvements in healthcare facilities drive the market, despite economic challenges and disparities in healthcare access.Tell us your focus area and get a customized research report.
Therapeutic Stents Market Analysis By Product
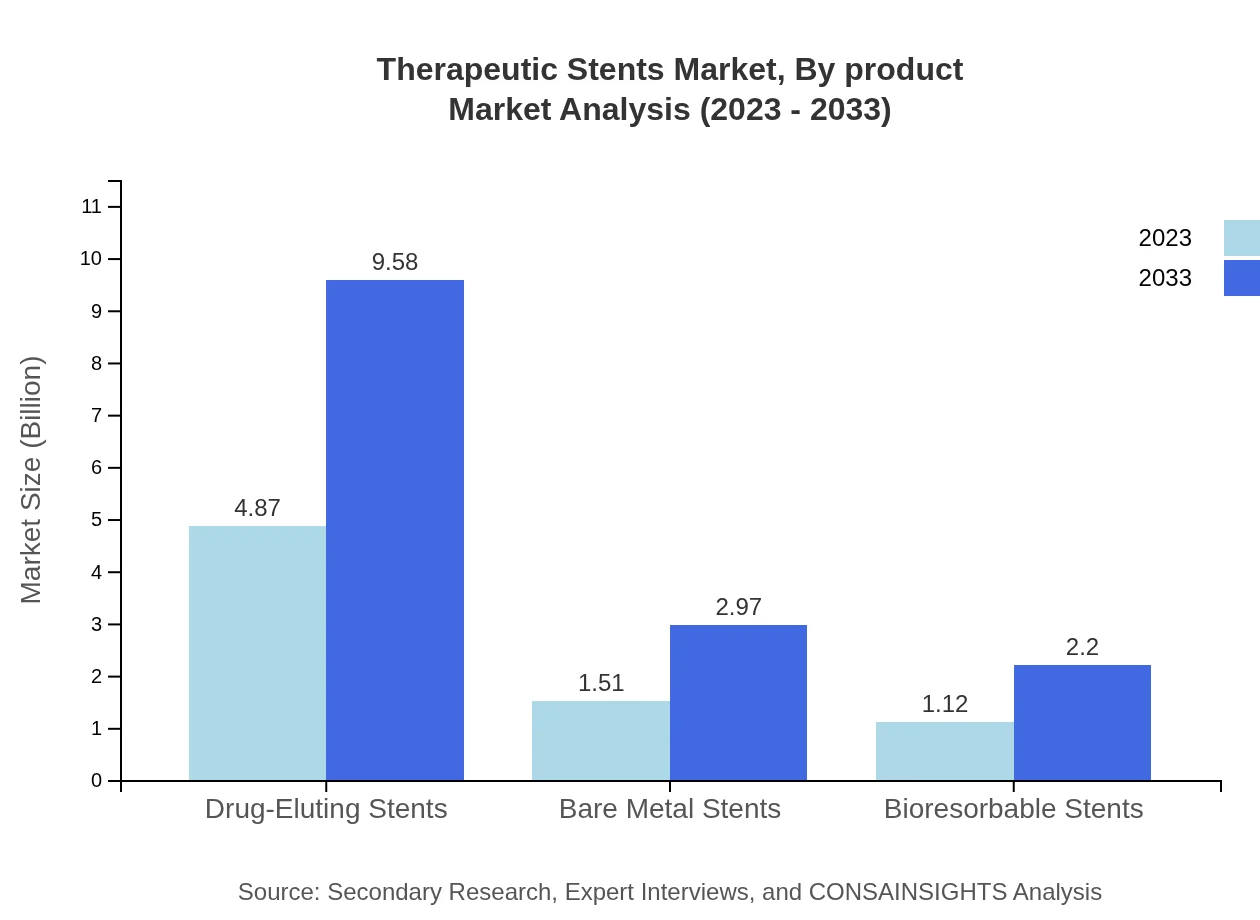
The therapeutic stents market by product showcases a robust performance, with drug-eluting stents leading the segment. In 2023, drug-eluting stents are projected to generate a market size of $4.87 billion, increasing to $9.58 billion by 2033, maintaining a market share of 64.96%. Bare-metal stents are valued at $1.51 billion in 2023 and expected to reach $2.97 billion in 2033, capturing a 20.13% market share. Bioresorbable stents, while smaller at $1.12 billion in 2023, are projected to grow to $2.20 billion by 2033, securing a 14.91% market share.
Therapeutic Stents Market Analysis By Indication
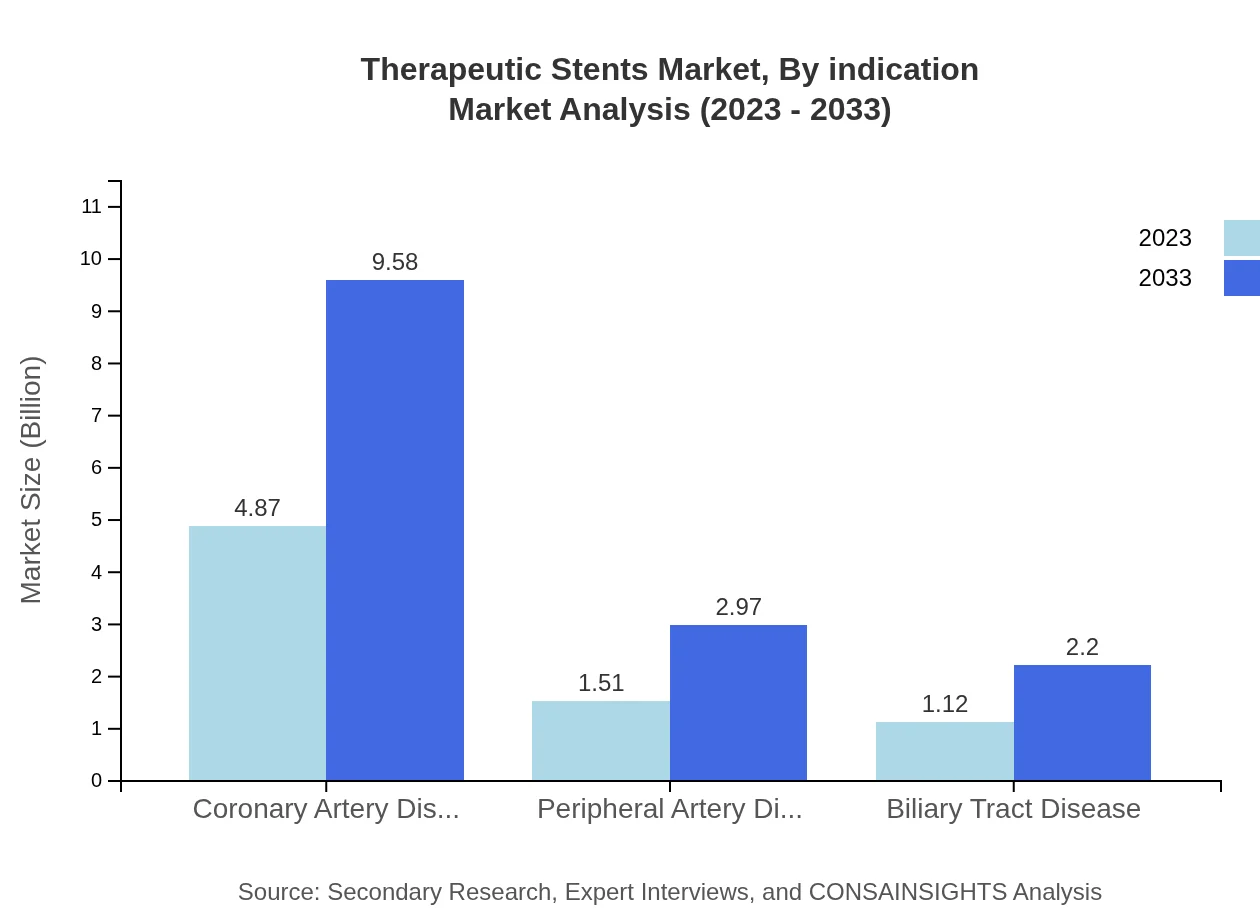
By indication, coronary artery disease stands as the dominant segment, valued at $4.87 billion in 2023 and projected to grow to $9.58 billion by 2033, holding a steady 64.96% market share. Peripheral artery disease, on the other hand, is expected to grow from $1.51 billion to $2.97 billion within the same period, capturing 20.13% of the market. Lastly, biliary tract disease begins at $1.12 billion and forecasted to reach $2.20 billion, maintaining a 14.91% share.
Therapeutic Stents Market Analysis By Technology
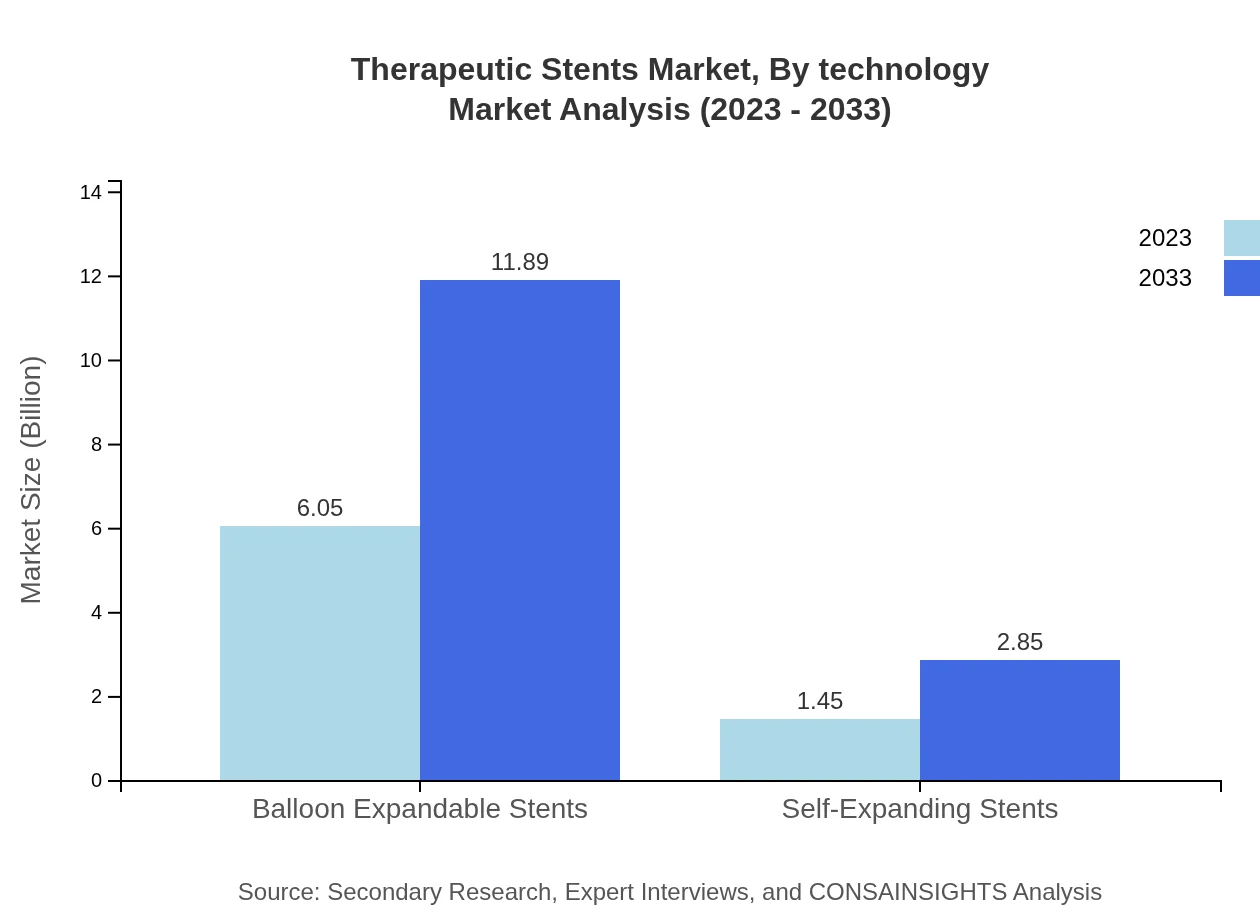
In terms of technology, balloon-expandable stents are expected to dominate, projected at $6.05 billion in 2023 and growing to $11.89 billion by 2033, holding a market share of 80.69%. Self-expanding stents, in contrast, will start at $1.45 billion in 2023 with anticipated growth to $2.85 billion, representing a 19.31% share of the market.
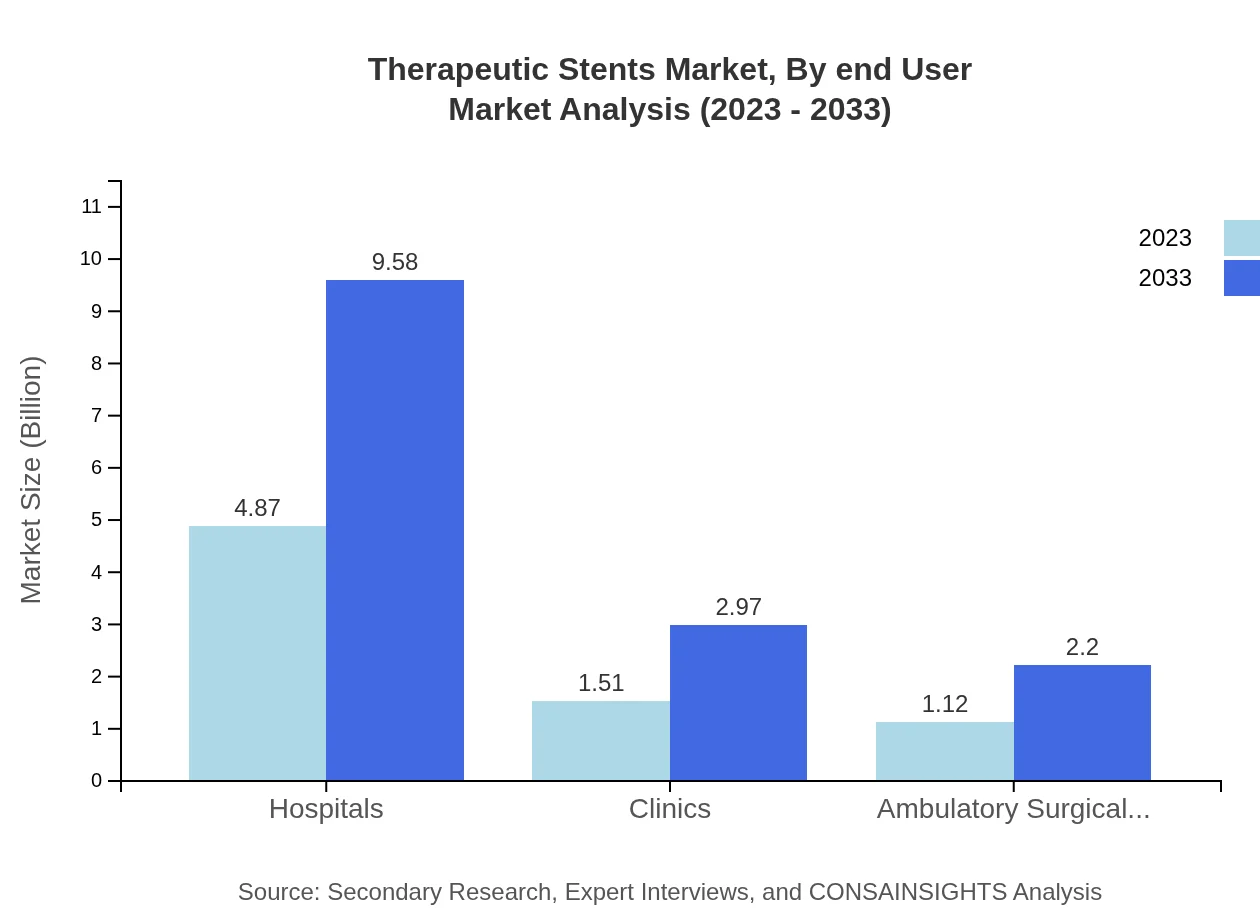
Therapeutic Stents Market Analysis By End User
The therapeutic stents market by end-user indicates that hospitals are the largest segment, valued at $4.87 billion in 2023 and expected to reach $9.58 billion by 2033, encompassing 64.96% of the market. Clinics are projected to increase from $1.51 billion to $2.97 billion, holding a 20.13% share, while ambulatory surgical centers will grow from $1.12 billion to $2.20 billion, acquiring 14.91% market share.
Therapeutic Stents Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Therapeutic Stents Industry
Abbott Laboratories:
A leader in medical devices, Abbott is renowned for its innovative drug-eluting stents and commitment to advancing healthcare. Their integrated solutions for coronary artery disease significantly enhance patient outcomes.Boston Scientific Corporation:
A global medical device manufacturer, Boston Scientific excels in therapeutic stents, offering a wide range of vascular interventions with a focus on quality and innovation in treatment options.Medtronic plc:
Known for its comprehensive portfolio in cardiovascular medicine, Medtronic is a pivotal player in the therapeutic stents market, contributing advanced technologies to improve vascular therapies.Cordis (a Cardinal Health company):
Cordis is renowned for its innovative endovascular solutions, particularly in the stent market, with a focus on improving procedural efficiency and patient recovery.Terumo Corporation:
A well-established name in the medical field, Terumo is recognized for its high-quality stenting solutions, emphasizing safety and effectiveness in cardiovascular interventions.We're grateful to work with incredible clients.









FAQs
What is the market size of therapeutic Stents?
The therapeutic stents market is valued at approximately $7.5 billion in 2023 and is projected to grow at a CAGR of 6.8%, reaching significant market size by 2033.
What are the key market players or companies in this therapeutic Stents industry?
Key players in the therapeutic stents industry include large medical device manufacturers such as Boston Scientific, Abbott Laboratories, and Medtronic, which dominate with innovative product offerings and strong market presence.
What are the primary factors driving the growth in the therapeutic stents industry?
Growth factors include rising incidences of coronary and peripheral artery diseases, technological advancements in stent design, and an increasing aging population leading to higher healthcare spending.
Which region is the fastest Growing in the therapeutic Stents?
The Asia-Pacific region is experiencing the fastest growth in the therapeutic stents market, expanding from $1.53 billion in 2023 to $3.01 billion in 2033, driven by increased healthcare access and investment.
Does ConsaInsights provide customized market report data for the therapeutic Stents industry?
Yes, ConsaInsights offers customized market reports tailored to client specifications, allowing stakeholders to access unique data insights relevant to their business needs in the therapeutic stents industry.
What deliverables can I expect from this therapeutic Stents market research project?
Expect deliverables such as comprehensive market analysis reports, segmentation insights, competitor analysis, trend forecasts, and strategic recommendations for stakeholders in the therapeutic stents market.
What are the market trends of therapeutic Stents?
Market trends include a shift towards drug-eluting and bioresorbable stents, increasing minimally invasive procedures, and advancements in stent technology, enhancing patient outcomes and driving adoption.