Therapeutic Vaccine Market Report
Published Date: 31 January 2026 | Report Code: therapeutic-vaccine
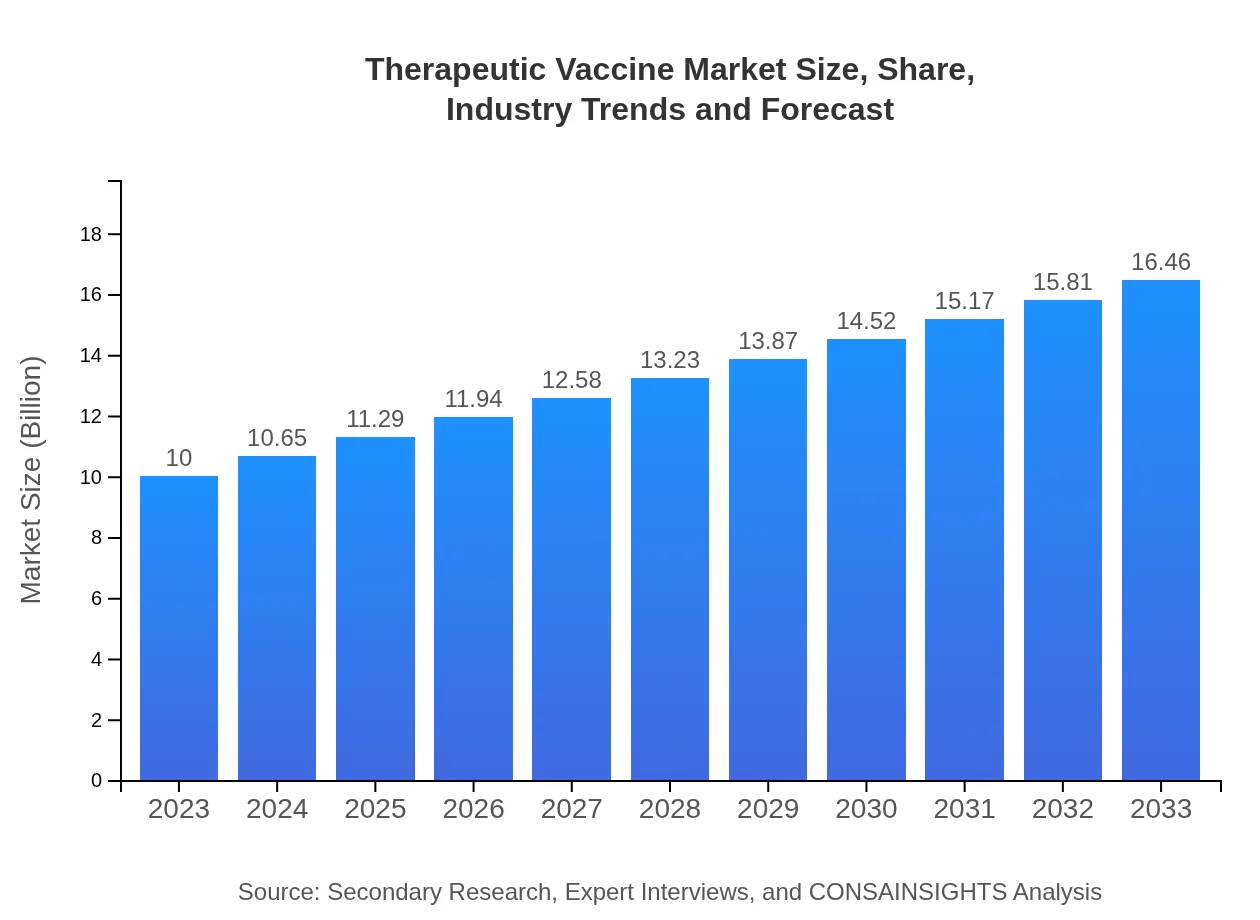
Therapeutic Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Therapeutic Vaccine market from 2023 to 2033, focusing on market trends, size, regional insights, and competitive landscape alongside technological advancements and future forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $16.46 Billion |
| Top Companies | Moderna, Inc., Pfizer Inc., GlaxoSmithKline (GSK), Sanofi |
| Last Modified Date | 31 January 2026 |
Therapeutic Vaccine Market Overview
Customize Therapeutic Vaccine Market Report market research report
- ✔ Get in-depth analysis of Therapeutic Vaccine market size, growth, and forecasts.
- ✔ Understand Therapeutic Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Therapeutic Vaccine
What is the Market Size & CAGR of Therapeutic Vaccine market in 2033?
Therapeutic Vaccine Industry Analysis
Therapeutic Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Therapeutic Vaccine Market Analysis Report by Region
Europe Therapeutic Vaccine Market Report:
The European market for Therapeutic Vaccines is estimated to be USD 3.29 billion in 2023, projected to expand to USD 5.42 billion by 2033. The region is characterized by strong government support for healthcare innovations and a rising prevalence of chronic diseases which demand effective therapeutic solutions.Asia Pacific Therapeutic Vaccine Market Report:
In 2023, the Asia Pacific Therapeutic Vaccine market is valued at USD 1.83 billion and is projected to reach USD 3.01 billion by 2033. This region is witnessing rapid advancements in healthcare infrastructure and increasing investments from both government and private sectors, making it a significant market for therapeutic vaccines.North America Therapeutic Vaccine Market Report:
North America holds a substantial share of the Therapeutic Vaccine market, with a valuation of USD 3.43 billion in 2023 and an expected growth to USD 5.64 billion by 2033. Key factors contributing to growth include a robust healthcare infrastructure, high R&D investments, and an increasing number of approvals for therapeutic products.South America Therapeutic Vaccine Market Report:
The South American Therapeutic Vaccine market was valued at USD 0.91 billion in 2023 and is expected to grow to USD 1.50 billion by 2033. Demand is intensifying for modern therapeutic solutions, especially in addressing endemic diseases, which propels the market forward in this region.Middle East & Africa Therapeutic Vaccine Market Report:
In the Middle East and Africa, the Therapeutic Vaccine market is valued at USD 0.53 billion in 2023, anticipated to grow to USD 0.88 billion by 2033. The expansion is driven by increasing healthcare awareness and ongoing initiatives aimed at improving vaccination programs.Tell us your focus area and get a customized research report.
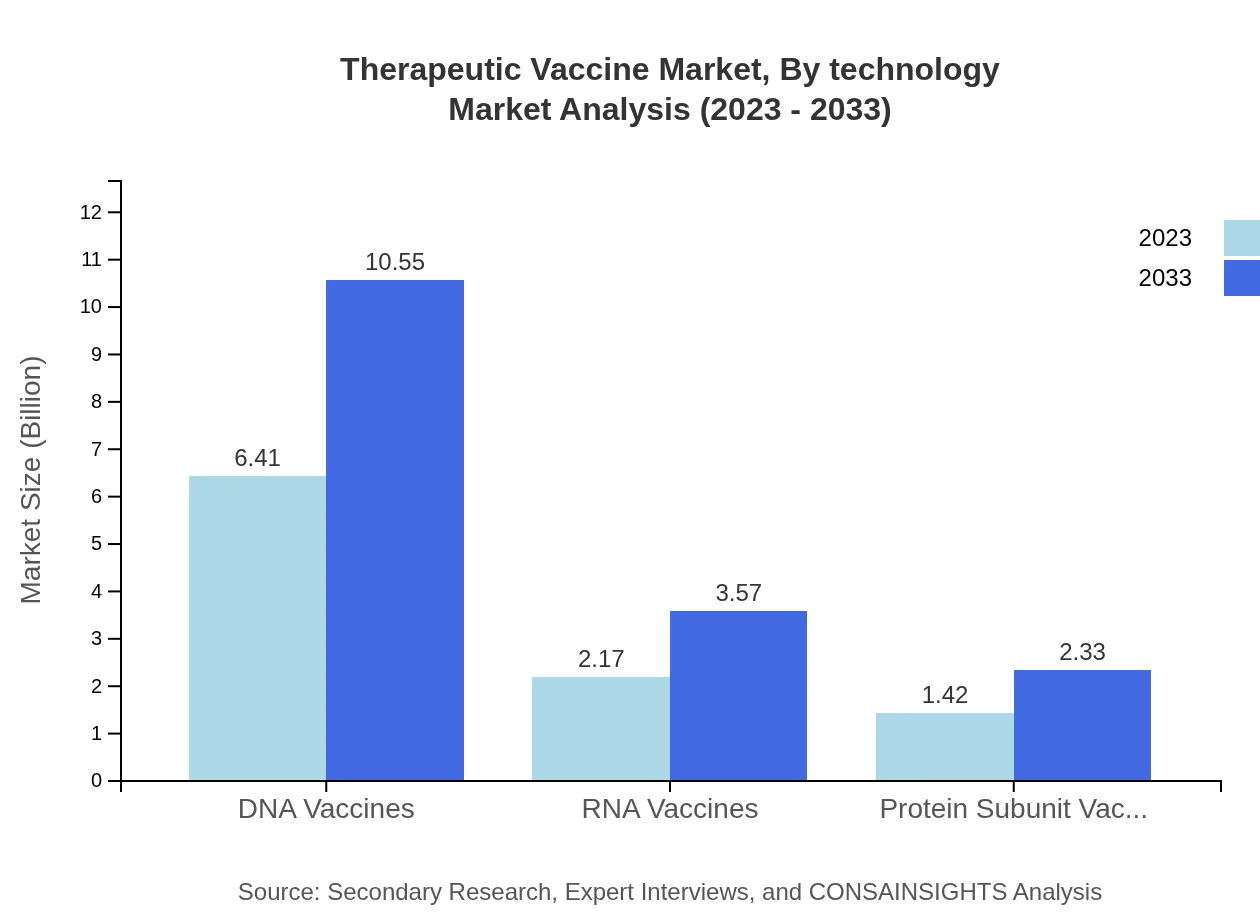
Therapeutic Vaccine Market Analysis By Technology
The Therapeutic Vaccine market operates on various technologies, prominently featuring DNA and RNA vaccines, which accounted for USD 6.41 billion in 2023, projected to increase to USD 10.55 billion by 2033, showing significant market share due to their innovative mechanisms in combating diseases. RNA vaccines are gradually gaining a larger market share, moving from USD 2.17 billion in 2023 to USD 3.57 billion by 2033.
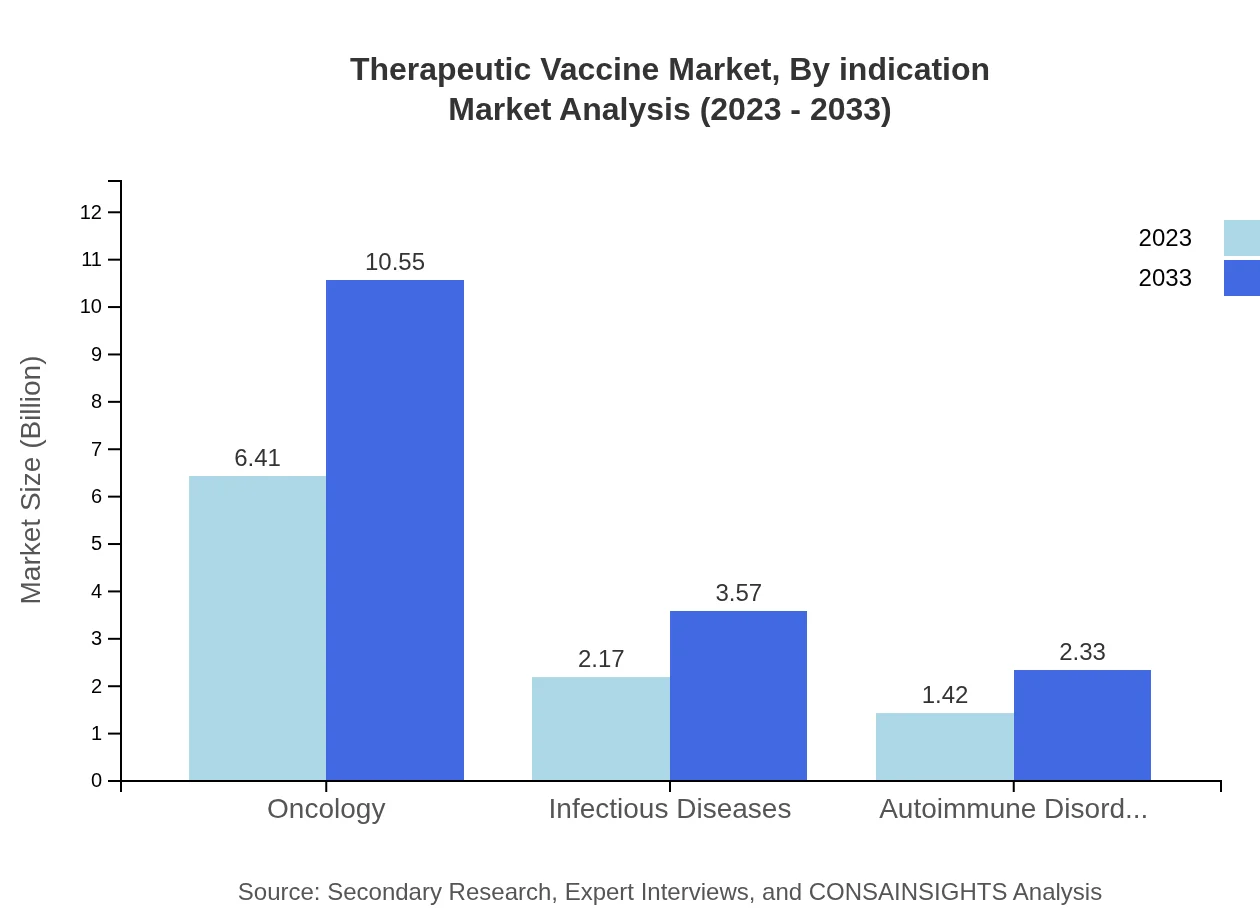
Therapeutic Vaccine Market Analysis By Indication
In terms of indication, oncology vaccines dominate the market, valued at USD 6.41 billion in 2023 and expected to reach USD 10.55 billion by 2033. Infectious disease therapeutic vaccines are also significant, achieving USD 2.17 billion in 2023 and projected to grow to USD 3.57 billion by 2033. Autoimmune disorder vaccines represent a growing segment as well, with a rise from USD 1.42 billion to USD 2.33 billion by 2033.
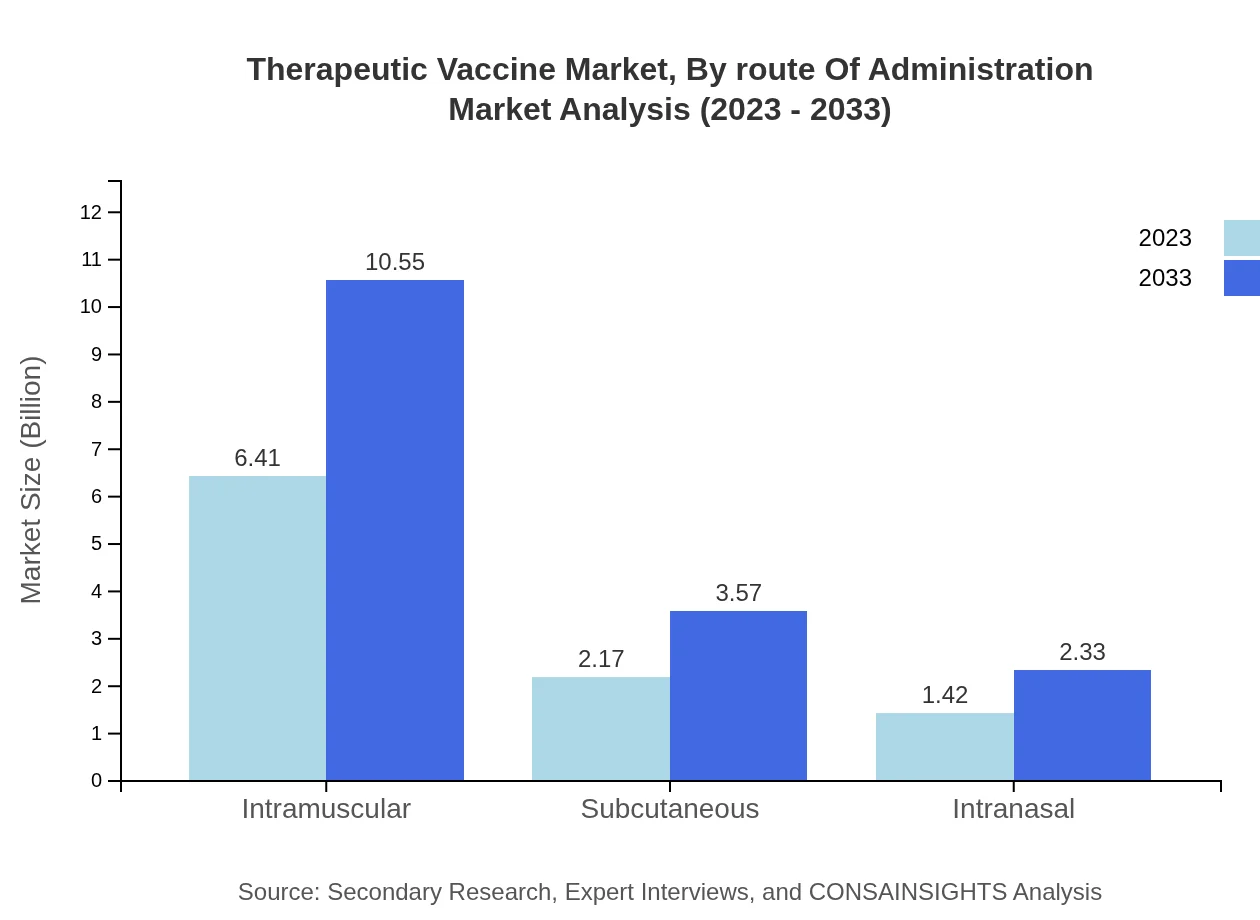
Therapeutic Vaccine Market Analysis By Route Of Administration
Various routes of administration including intramuscular, subcutaneous, and intranasal are utilized within the Therapeutic Vaccine market. Intramuscular vaccines are projected to remain the leading route, expanding from USD 6.41 billion in 2023 to USD 10.55 billion by 2033. Subcutaneous routes are also growing in importance, expecting a rise from USD 2.17 billion to USD 3.57 billion in the same period.
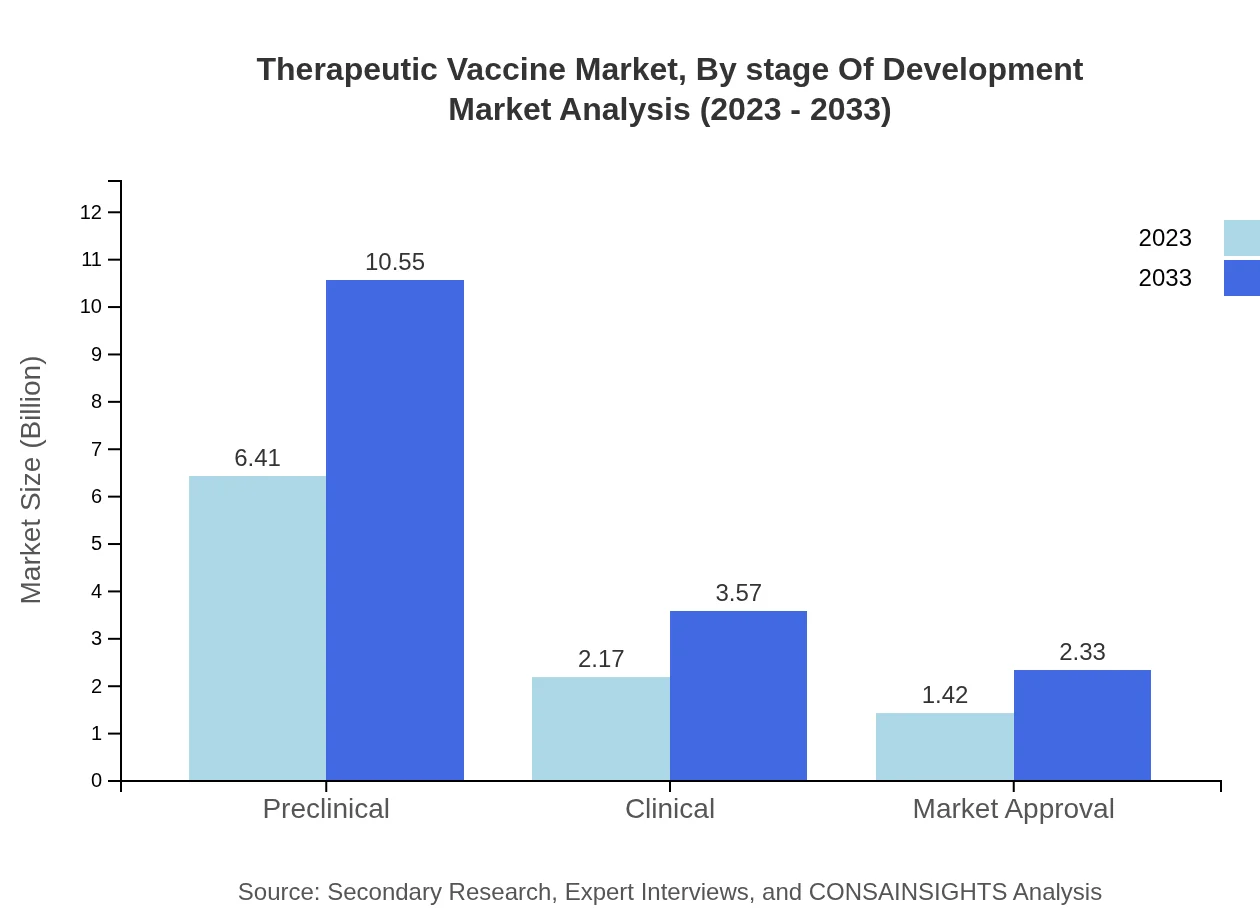
Therapeutic Vaccine Market Analysis By Stage Of Development
Market segments by stage of development show that Preclinical stage vaccines dominate the landscape, expected to grow from USD 6.41 billion to USD 10.55 billion. Clinical stage assessments are equally crucial, moving from USD 2.17 billion to USD 3.57 billion in 2033. Furthermore, vaccines ready for market approval represent a vital segment, expanding from USD 1.42 billion to USD 2.33 billion.
Therapeutic Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Therapeutic Vaccine Industry
Moderna, Inc.:
Moderna is a pioneer in mRNA technology, focusing on innovative vaccines that deliver transformative solutions for infectious diseases and cancers.Pfizer Inc.:
Pfizer, a key player in the vaccines market, is well-known for its contributions to COVID-19 vaccine development and its ongoing research in therapeutic vaccines across various indications.GlaxoSmithKline (GSK):
GSK is a major player in therapeutic vaccines, focusing on extensive research to innovate vaccines against cancer and infectious diseases.Sanofi:
Sanofi invests heavily in R&D and has a diverse pipeline of therapeutic vaccines, particularly focusing on autoimmunity and oncology.We're grateful to work with incredible clients.









FAQs
What is the market size of therapeutic Vaccine?
The therapeutic vaccine market is projected to reach approximately $10 billion by 2033, growing at a CAGR of 5%. This growth reflects increased research and development efforts in treatments for various diseases.
What are the key market players or companies in the therapeutic vaccine industry?
Key players in the therapeutic vaccine market include major pharmaceutical firms like Merck, Pfizer, and Novartis, which are heavily investing in R&D and partnerships to innovate and expand their product offerings.
What are the primary factors driving the growth in the therapeutic vaccine industry?
Growth drivers include rising prevalence of chronic diseases, advancements in vaccine technology, increased healthcare investments, and a growing focus on preventive health measures, fostering a robust demand for therapeutic vaccines.
Which region is the fastest Growing in the therapeutic vaccine market?
The Asia Pacific region is the fastest-growing market for therapeutic vaccines, expected to increase from $1.83 billion in 2023 to $3.01 billion by 2033, driven by expanding healthcare infrastructure and innovation.
Does ConsaInsights provide customized market report data for the therapeutic vaccine industry?
Yes, ConsaInsights offers customized market report data tailored to the therapeutic vaccine industry, allowing clients to access specific insights relevant to their needs and strategic objectives.
What deliverables can I expect from this therapeutic vaccine market research project?
Deliverables include comprehensive market analysis reports, insights on trends and forecasts, competitive landscape assessments, and segmentation analyses across various therapeutic vaccine categories.
What are the market trends of therapeutic vaccine?
Key trends include a rising focus on oncology therapeutic vaccines, shifts towards personalized medicine, and increasing investments in DNA and RNA vaccine technology, enhancing market dynamism.