Thrombosis Drugs Market Report
Published Date: 31 January 2026 | Report Code: thrombosis-drugs
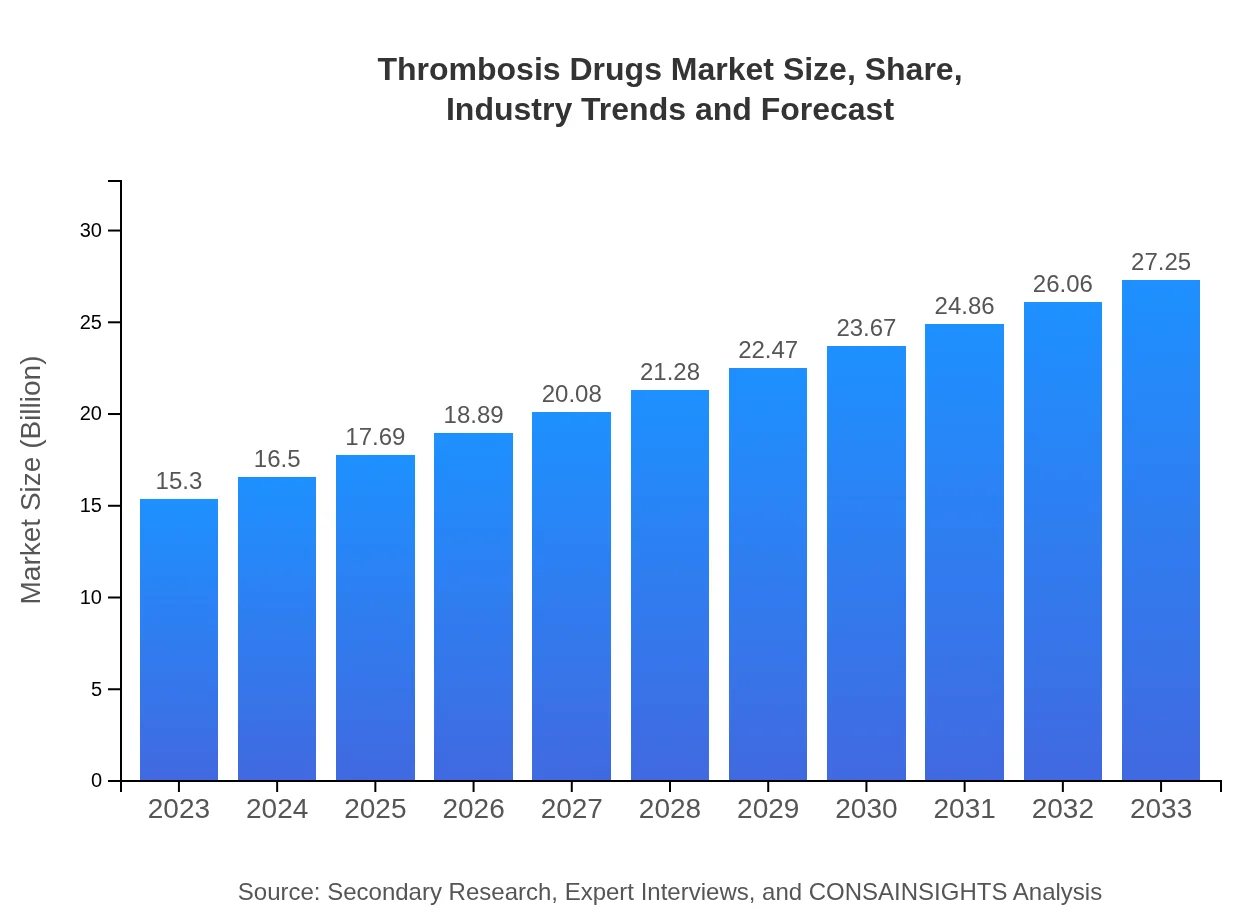
Thrombosis Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the thrombosis drugs market from 2023 to 2033, covering essential insights into market trends, regional analyses, and projections for growth and innovation within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $15.30 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $27.25 Billion |
| Top Companies | Boehringer Ingelheim, AstraZeneca, Bayer AG, Johnson & Johnson, Sanofi |
| Last Modified Date | 31 January 2026 |
Thrombosis Drugs Market Overview
Customize Thrombosis Drugs Market Report market research report
- ✔ Get in-depth analysis of Thrombosis Drugs market size, growth, and forecasts.
- ✔ Understand Thrombosis Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Thrombosis Drugs
What is the Market Size & CAGR of Thrombosis Drugs market in 2023?
Thrombosis Drugs Industry Analysis
Thrombosis Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Thrombosis Drugs Market Analysis Report by Region
Europe Thrombosis Drugs Market Report:
With a market value of $4.77 billion in 2023, Europe is expected to reach $8.49 billion by 2033. The growth is stimulated by advanced healthcare systems, significant R&D investment, and a focus on innovative treatment solutions.Asia Pacific Thrombosis Drugs Market Report:
In 2023, the Asia Pacific thrombosis drugs market is valued at $3.10 billion and is projected to reach $5.51 billion by 2033. The growth is driven by an aging population, increased healthcare investments, and rising awareness of cardiovascular diseases and associated treatments.North America Thrombosis Drugs Market Report:
The North American thrombosis drugs market is projected to grow from $5.00 billion in 2023 to $8.91 billion by 2033. High prevalence rates of thromboembolic disorders and extensive research activities in this region are key contributors to this growth.South America Thrombosis Drugs Market Report:
The South American market for thrombosis drugs stands at $0.94 billion in 2023, expected to grow to $1.68 billion by 2033. The expansion is largely attributed to improving healthcare infrastructure and increased accessibility to medical treatments.Middle East & Africa Thrombosis Drugs Market Report:
The Middle East and Africa thrombosis drugs market is anticipated to grow from $1.50 billion in 2023 to $2.67 billion by 2033. Rapid urbanization and growing health awareness are essential factors supporting this upward trend.Tell us your focus area and get a customized research report.
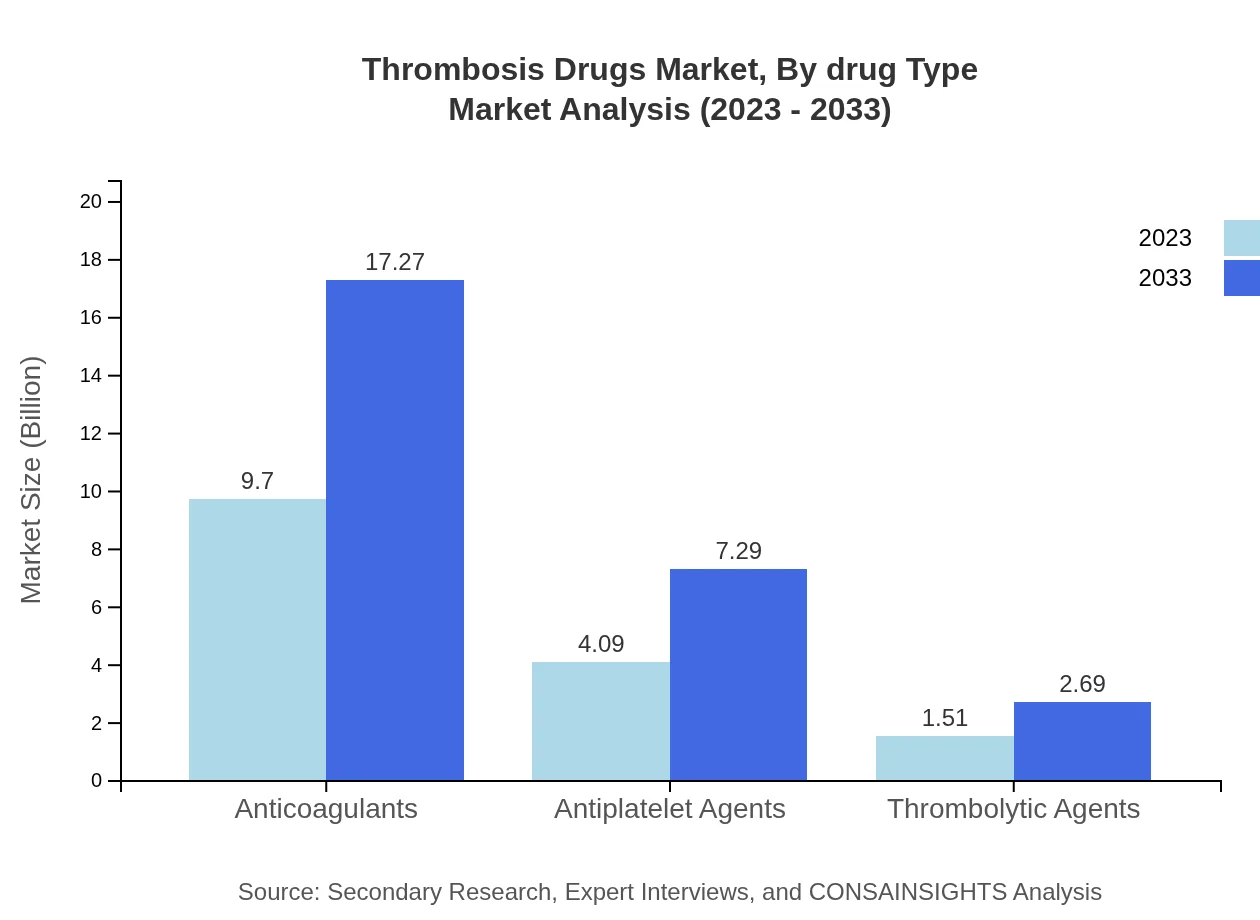
Thrombosis Drugs Market Analysis By Drug Type
By drug type, the market sees considerable demand for anticoagulants, which were valued at $9.70 billion in 2023 and are projected to reach $17.27 billion by 2033. Antiplatelet agents, valued at $4.09 billion in 2023, will also experience growth, reaching $7.29 billion by 2033. Thrombolytic agents represent a smaller segment, with size estimates at $1.51 billion and projections of $2.69 billion by 2033.
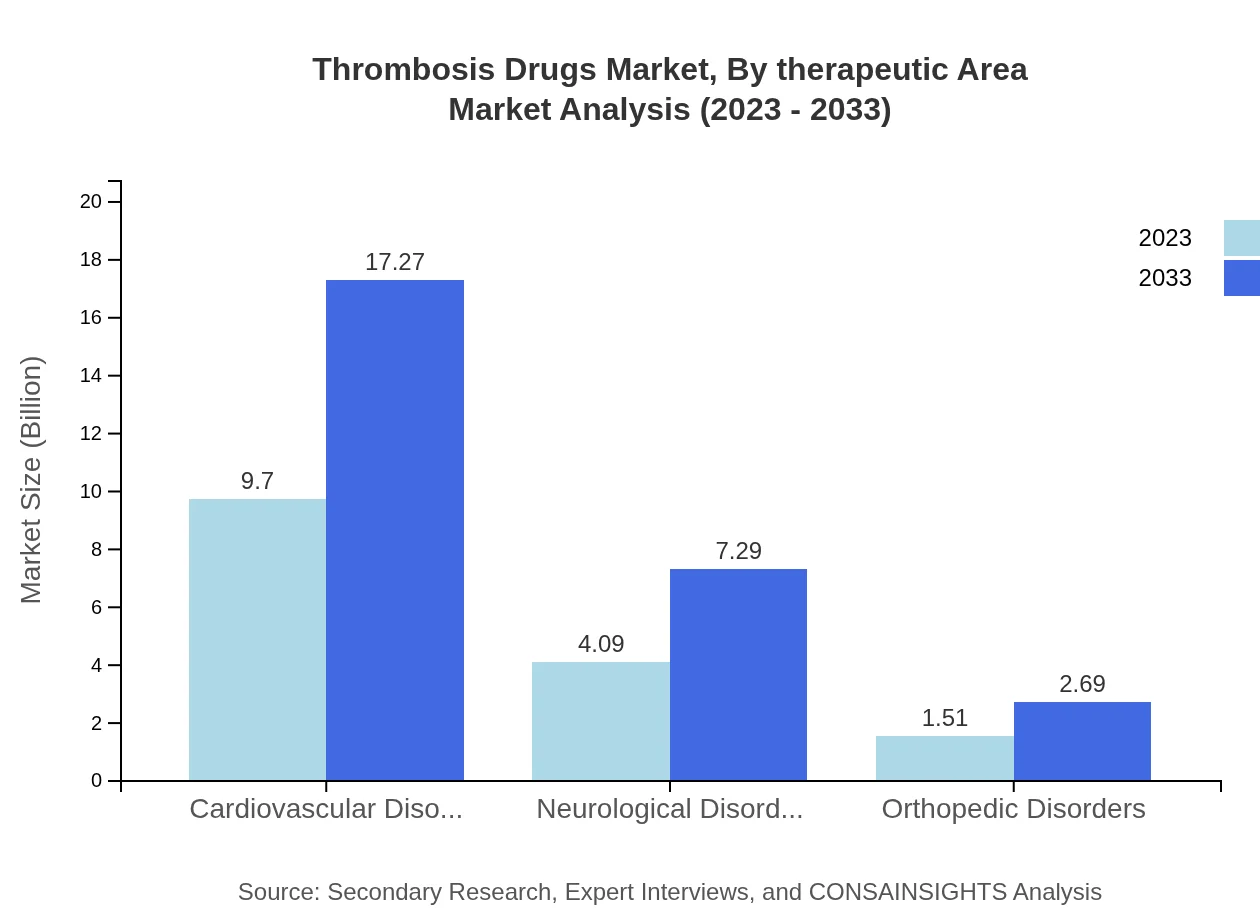
Thrombosis Drugs Market Analysis By Therapeutic Area
In therapeutic areas, cardiovascular disorders dominate the market, accounting for $9.70 billion in 2023, with a projected growth to $17.27 billion by 2033. Neurological disorders and orthopedic disorders have also shown growth potential, projected at $4.09 billion and $1.51 billion in 2023 respectively, growing to $7.29 billion and $2.69 billion by 2033.
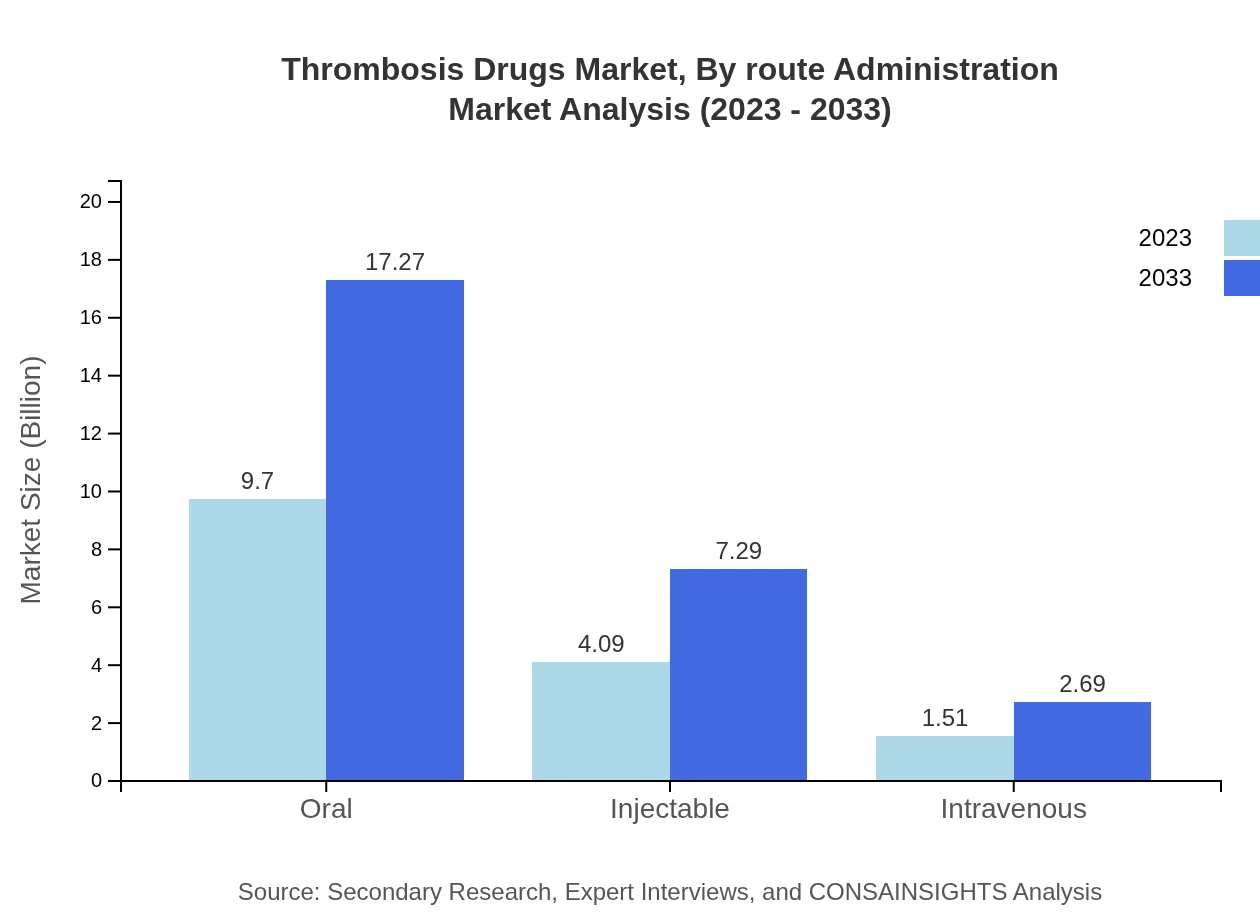
Thrombosis Drugs Market Analysis By Route Administration
On evaluating routes of administration, oral drugs are notable leaders, with a market size projected to increase from $9.70 billion in 2023 to $17.27 billion in the forecast period. Injectable formulations and intravenous routes are also growing, respectively estimated at $4.09 billion and $1.51 billion in 2023, with expected growth to $7.29 billion and $2.69 billion by 2033.
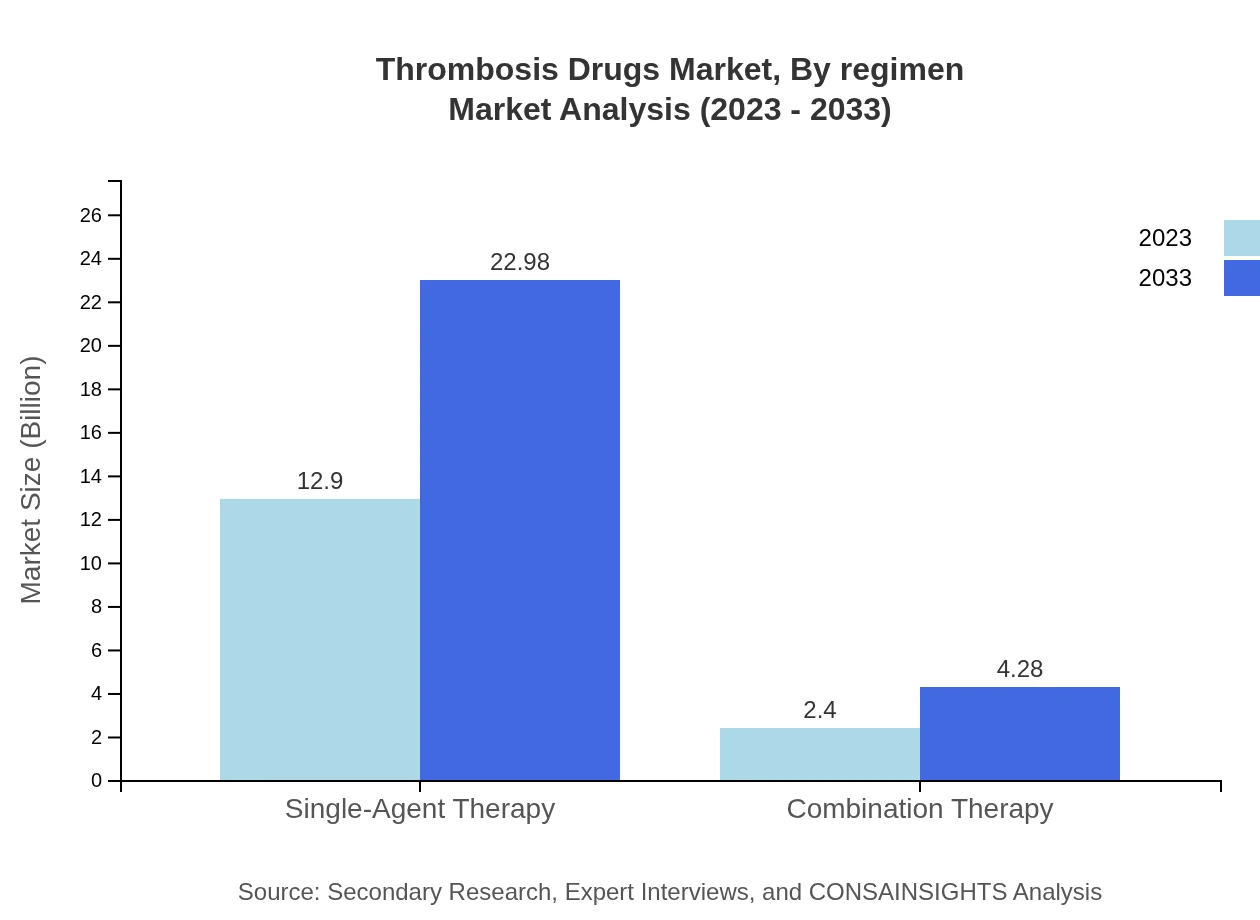
Thrombosis Drugs Market Analysis By Regimen
Single-agent therapy holds the majority share in the thrombosis market with a size of $12.90 billion in 2023, anticipated to reach $22.98 billion by 2033. Combination therapy is expected to grow from $2.40 billion to $4.28 billion in the same timeframe, affirming its role in comprehensive treatment approaches.
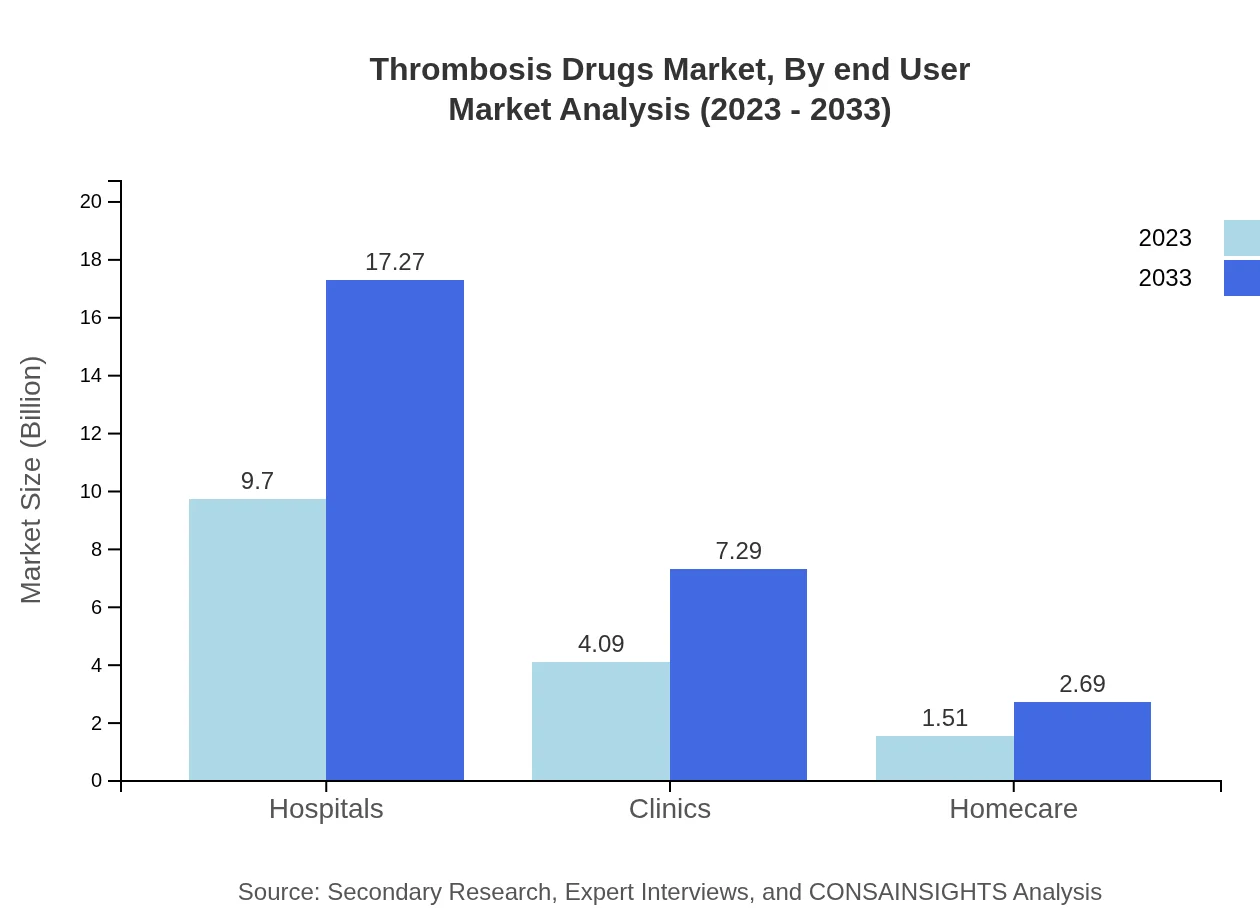
Thrombosis Drugs Market Analysis By End User
Hospitals are the primary end-users, holding a market share of $9.70 billion in 2023, increasing to $17.27 billion by 2033. Clinics and homecare settings also constitute vital constituents, with sizes of $4.09 billion and $1.51 billion in 2023, projected to grow to $7.29 billion and $2.69 billion respectively by 2033.
Thrombosis Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Thrombosis Drugs Industry
Boehringer Ingelheim:
A global leader in the cardiovascular therapeutic area, focusing on innovative thrombolytic and anticoagulant solutions.AstraZeneca:
Known for its extensive pharmaceutical portfolio, AstraZeneca offers advanced antiplatelet agents to enhance patient outcomes.Bayer AG:
A pioneer in anticoagulant research, Bayer specializes in cutting-edge drug development for thrombosis prevention and treatment.Johnson & Johnson:
This company provides a robust range of thrombosis therapies along with comprehensive healthcare solutions.Sanofi:
Sanofi focuses on providing effective anticoagulants and contributes significantly to R&D in this life-saving therapy area.We're grateful to work with incredible clients.









FAQs
What is the market size of thrombosis Drugs?
The thrombosis-drugs market is estimated at $15.3 billion in 2023, with a projected CAGR of 5.8% through 2033, indicating significant potential for growth and expansion in therapeutic applications.
What are the key market players or companies in the thrombosis Drugs industry?
Key players in the thrombosis-drugs market include major pharmaceutical companies that specialize in anticoagulants, antiplatelet agents, and thrombolytic agents, focusing on innovative treatments and expanding their market reach globally.
What are the primary factors driving the growth in the thrombosis Drugs industry?
Growth in the thrombosis-drugs industry is driven by increasing incidence of thromboembolic disorders, advancements in drug formulations, and rising awareness regarding the importance of thrombosis treatment among healthcare professionals and patients.
Which region is the fastest Growing in the thrombosis Drugs market?
Asia-Pacific is the fastest-growing region in the thrombosis-drugs market, projected to grow from $3.1 billion in 2023 to $5.51 billion by 2033, reflecting rising healthcare investments and increasing prevalence of cardiovascular diseases.
Does ConsaInsights provide customized market report data for the thrombosis Drugs industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the thrombosis-drugs industry, providing detailed insights and market analysis for better decision-making.
What deliverables can I expect from this thrombosis Drugs market research project?
Deliverables from the thrombosis-drugs market research project include comprehensive reports, market forecasts, competitor analysis, and actionable insights on trends, segments, and regional market dynamics.
What are the market trends of thrombosis Drugs?
Key trends in the thrombosis-drugs market include the rising adoption of single-agent therapy, advancements in targeted therapies, and a shift toward personalized medicine, enhancing treatment efficacy for patients worldwide.