Thyroid Cancer Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: thyroid-cancer-diagnostics
Thyroid Cancer Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Thyroid Cancer Diagnostics market, including future forecasts from 2023 to 2033, market size metrics, industry insights, regional breakdowns, and an overview of key players driving innovation in this field.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
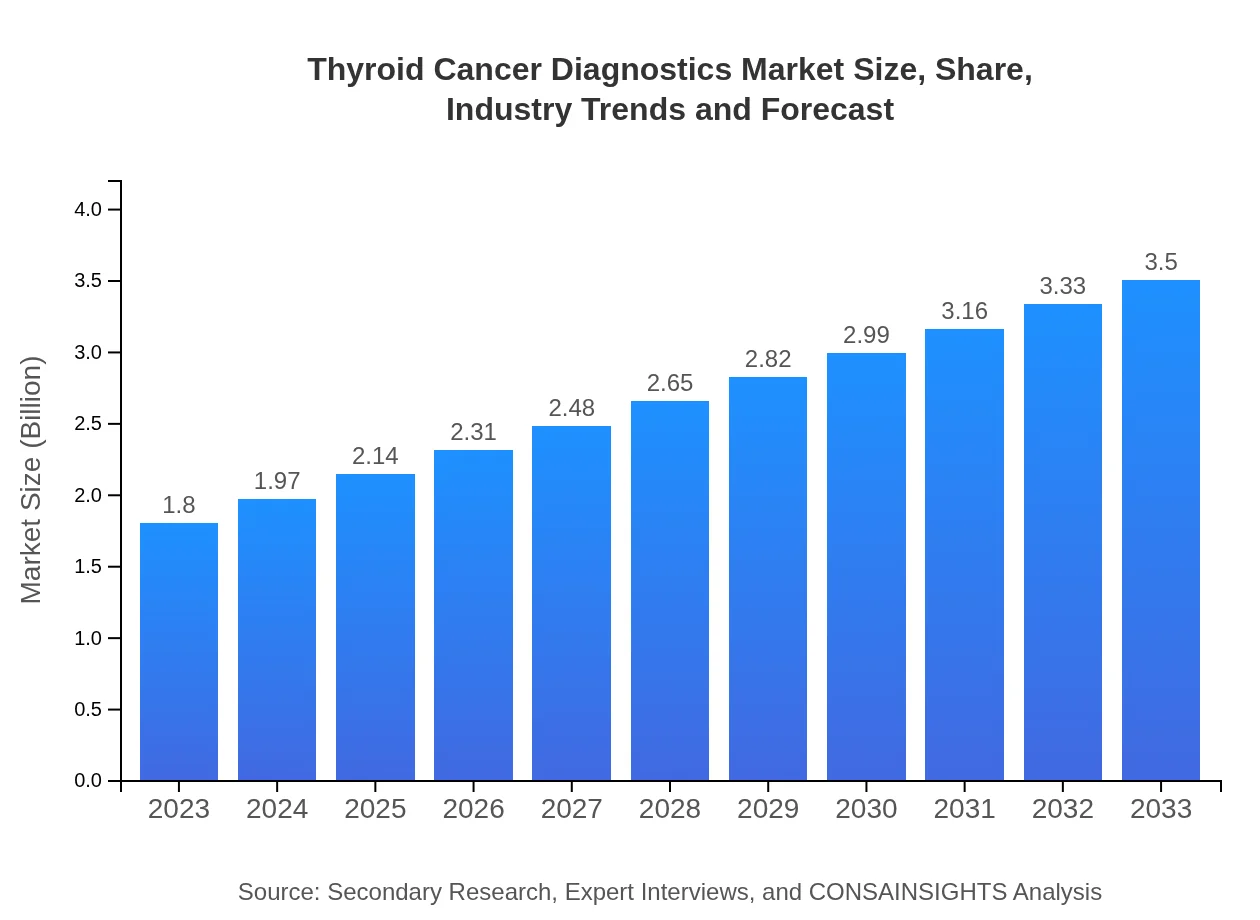
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $3.50 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Thyroid Cancer Diagnostics Market Overview
Customize Thyroid Cancer Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Thyroid Cancer Diagnostics market size, growth, and forecasts.
- ✔ Understand Thyroid Cancer Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Thyroid Cancer Diagnostics
What is the Market Size & CAGR of Thyroid Cancer Diagnostics market in 2023?
Thyroid Cancer Diagnostics Industry Analysis
Thyroid Cancer Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Thyroid Cancer Diagnostics Market Analysis Report by Region
Europe Thyroid Cancer Diagnostics Market Report:
Europe's market for thyroid cancer diagnostics is expected to increase from $0.63 billion in 2023 to $1.22 billion by 2033. The rise in thyroid cancer prevalence and advancements in diagnostic methods are key drivers in this region.Asia Pacific Thyroid Cancer Diagnostics Market Report:
The Asia Pacific region is expected to witness significant growth, with the market size projected to increase from $0.34 billion in 2023 to $0.66 billion by 2033. Increased healthcare spending, a large patient population, and rising awareness about thyroid health contribute to this growth.North America Thyroid Cancer Diagnostics Market Report:
The North American market is poised for continued growth, projected to rise from $0.61 billion in 2023 to $1.19 billion by 2033. This growth can be attributed to advanced healthcare technologies, increased research efforts, and the presence of major market players in this region.South America Thyroid Cancer Diagnostics Market Report:
In South America, the market for thyroid cancer diagnostics is anticipated to grow from $0.14 billion in 2023 to $0.27 billion by 2033. Factors such as improvements in healthcare infrastructure and access to new diagnostic technologies will drive this growth.Middle East & Africa Thyroid Cancer Diagnostics Market Report:
The Middle East and Africa region is projected to grow from $0.09 billion in 2023 to $0.17 billion by 2033. Increasing awareness about cancer detection and improving healthcare systems are boosting market growth.Tell us your focus area and get a customized research report.
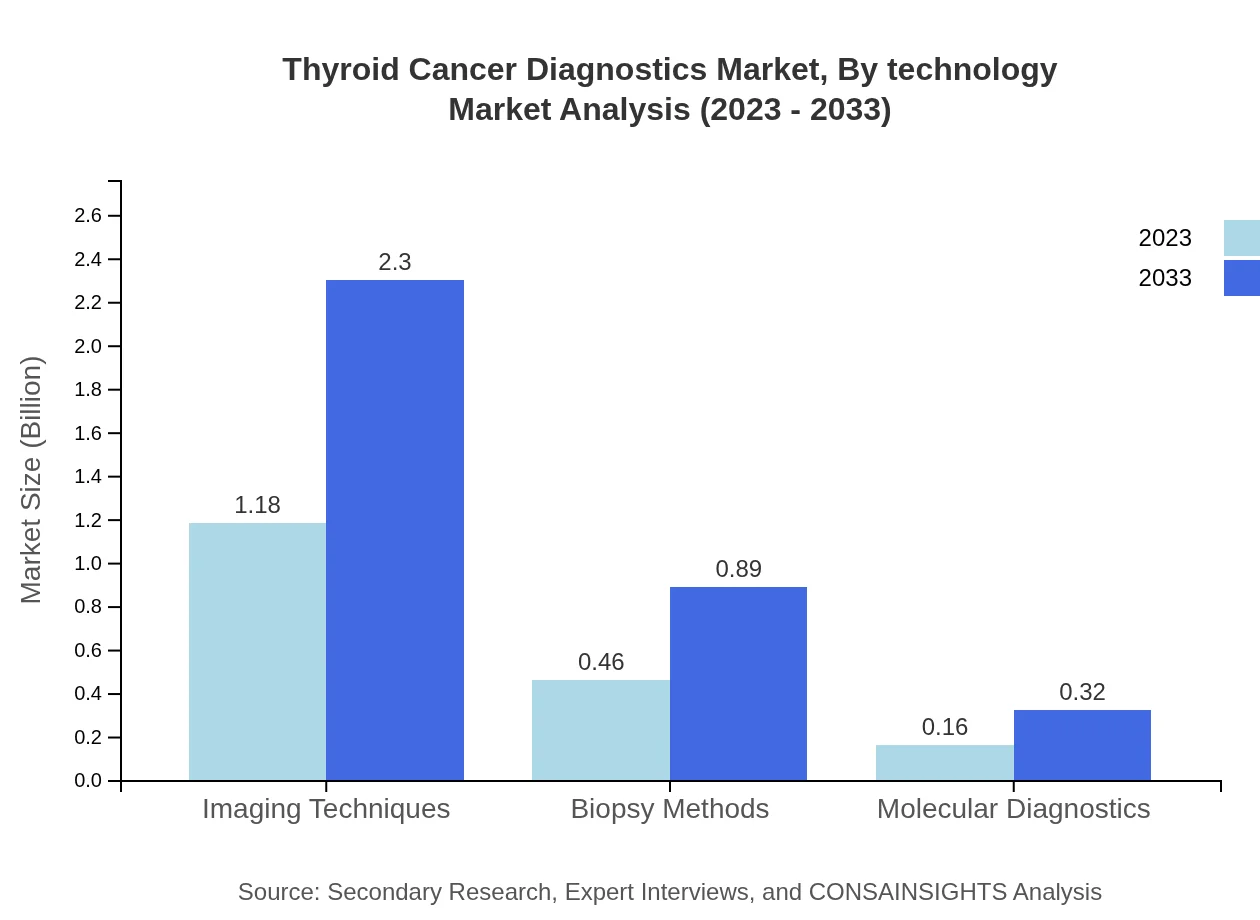
Thyroid Cancer Diagnostics Market Analysis By Technology
The market segmented by technology shows the dominance of imaging techniques, which accounted for $1.18 billion in 2023 and is expected to reach $2.30 billion by 2033. Other segments such as biopsy methods and molecular diagnostics also show significant growth, with respective projected increases reflecting the overall trend towards more precise diagnostic approaches.
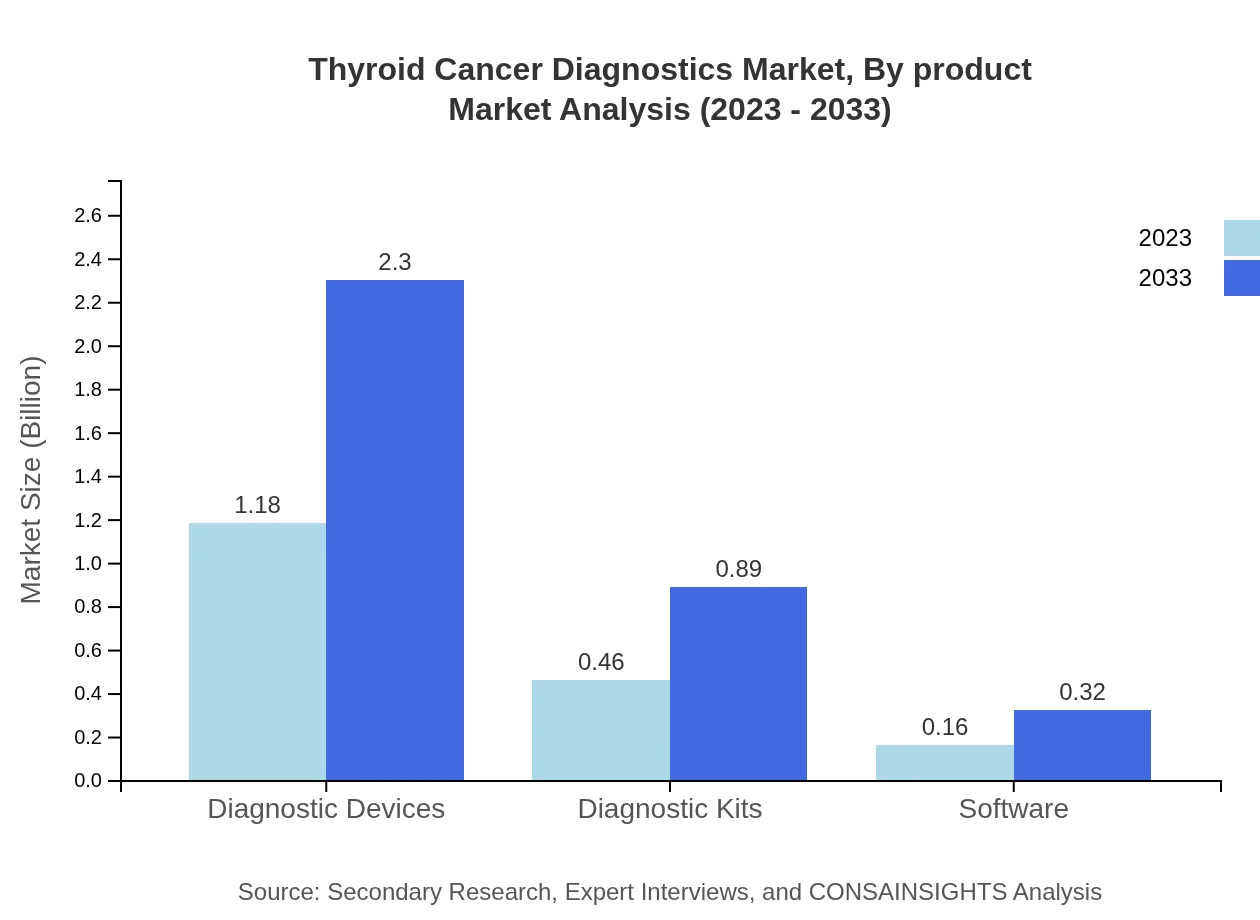
Thyroid Cancer Diagnostics Market Analysis By Product
The product analysis reveals that diagnostic devices hold the largest market share, with a size of $1.18 billion in 2023 and projected to reach $2.30 billion by 2033. Diagnostic kits follow closely, emphasizing the need for easy-to-use home testing options.
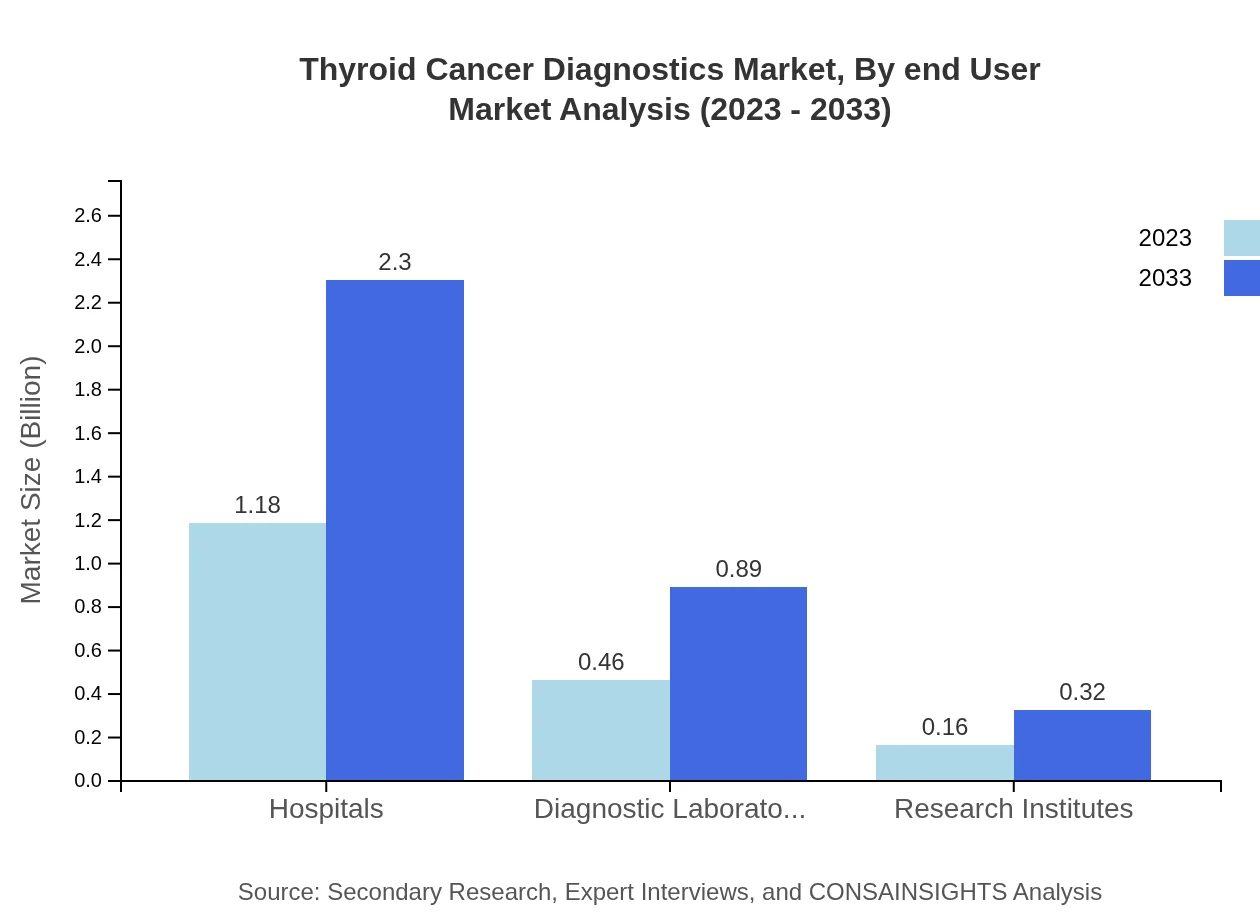
Thyroid Cancer Diagnostics Market Analysis By End User
Hospitals are the leading end-users in this market, accounting for a significant market share, with a size projected to grow from $1.18 billion in 2023 to $2.30 billion by 2033. Diagnostic laboratories and research institutes also contribute substantially to the growth of the market.
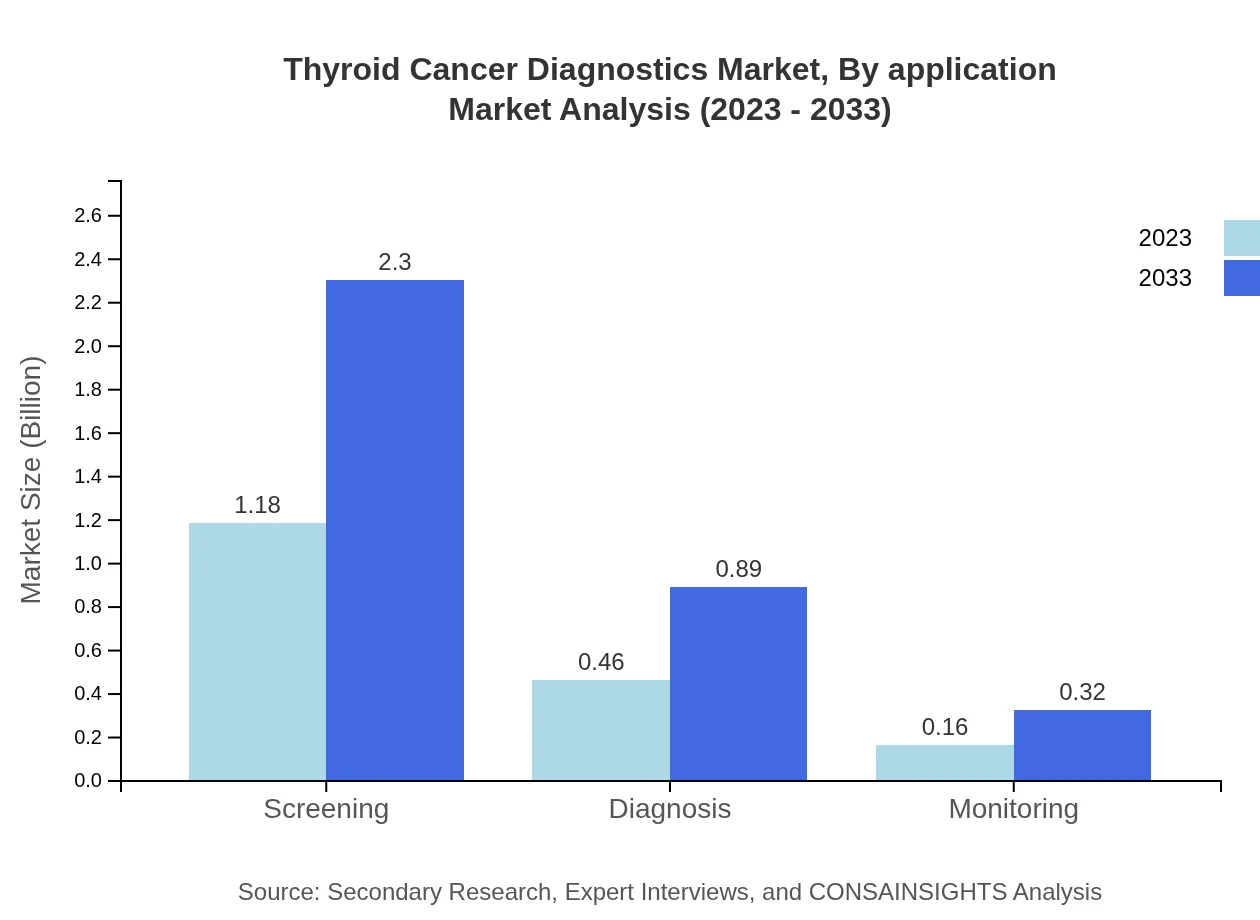
Thyroid Cancer Diagnostics Market Analysis By Application
The applications of diagnostics are varied, with screening and diagnosis holding substantial shares in the market. Screening is expected to dominate due to the increased focus on preventative healthcare, while diagnosis methods are innovating to improve accuracy.
Thyroid Cancer Diagnostics Market Analysis By Region
Global Thyroid Cancer Diagnostics Market, By Region Market Analysis (2023 - 2033)
Analyzing by region, the North America segment exhibits the highest growth potential, followed by Europe and Asia Pacific, due to the concentrated effort on healthcare improvements and innovative diagnostics in these areas.
Thyroid Cancer Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Thyroid Cancer Diagnostics Industry
Roche Diagnostics:
A leading player in the diagnostics space, Roche is known for its innovative laboratory diagnostics and has contributed significantly to thyroid cancer diagnostics through advanced testing solutions.Abbott Laboratories:
Abbott is recognized for its diagnostic tools that aid healthcare providers in accurately diagnosing thyroid cancer, with a strong focus on enhancing patient care through technology.Siemens Healthineers:
Siemens provides cutting-edge imaging technologies and diagnostics, playing a pivotal role in the advancements made in diagnosing thyroid cancers across diverse healthcare settings.Thermo Fisher Scientific:
Thermo Fisher specializes in molecular diagnostics and offers a wide array of tools aiding in the diagnosis and monitoring of thyroid cancer.We're grateful to work with incredible clients.









FAQs
What is the market size of thyroid cancer diagnostics?
The thyroid cancer diagnostics market is valued at $1.8 billion in 2023 and is projected to grow at a CAGR of 6.7% through 2033, showcasing strong growth potential in this critical healthcare sector.
What are the key market players or companies in the thyroid cancer diagnostics industry?
Key players in the thyroid cancer diagnostics industry include major companies specializing in medical devices, diagnostic kits, and software solutions tailored for accurate thyroid cancer diagnosis and management.
What are the primary factors driving the growth in the thyroid cancer diagnostics industry?
The growth in the thyroid cancer diagnostics industry is primarily driven by increasing thyroid cancer prevalence, advancements in diagnostic technologies, and a growing focus on early detection methods among healthcare professionals.
Which region is the fastest Growing in the thyroid cancer diagnostics?
The fastest-growing region in the thyroid cancer diagnostics market is Europe, projected to grow from $0.63 billion in 2023 to $1.22 billion by 2033, reflecting increasing awareness and technological advancements in the region.
Does ConsaInsights provide customized market report data for the thyroid cancer diagnostics industry?
Yes, ConsaInsights provides tailored market report data for the thyroid cancer diagnostics industry, allowing clients to obtain insights specific to their needs and strategic interests.
What deliverables can I expect from this thyroid cancer diagnostics market research project?
Deliverables include comprehensive market analyses, segment data, competitive landscapes, key trends, growth projections, and actionable insights tailored to the thyroid cancer diagnostics sector.
What are the market trends of thyroid cancer diagnostics?
Current market trends indicate a shift towards advanced diagnostic technologies, increased investments in research, and a growing emphasis on personalized medicine in thyroid cancer diagnostics.