Thyroid Function Tests Devices Market Report
Published Date: 31 January 2026 | Report Code: thyroid-function-tests-devices
Thyroid Function Tests Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Thyroid Function Tests Devices market, offering insights into current trends, market size, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
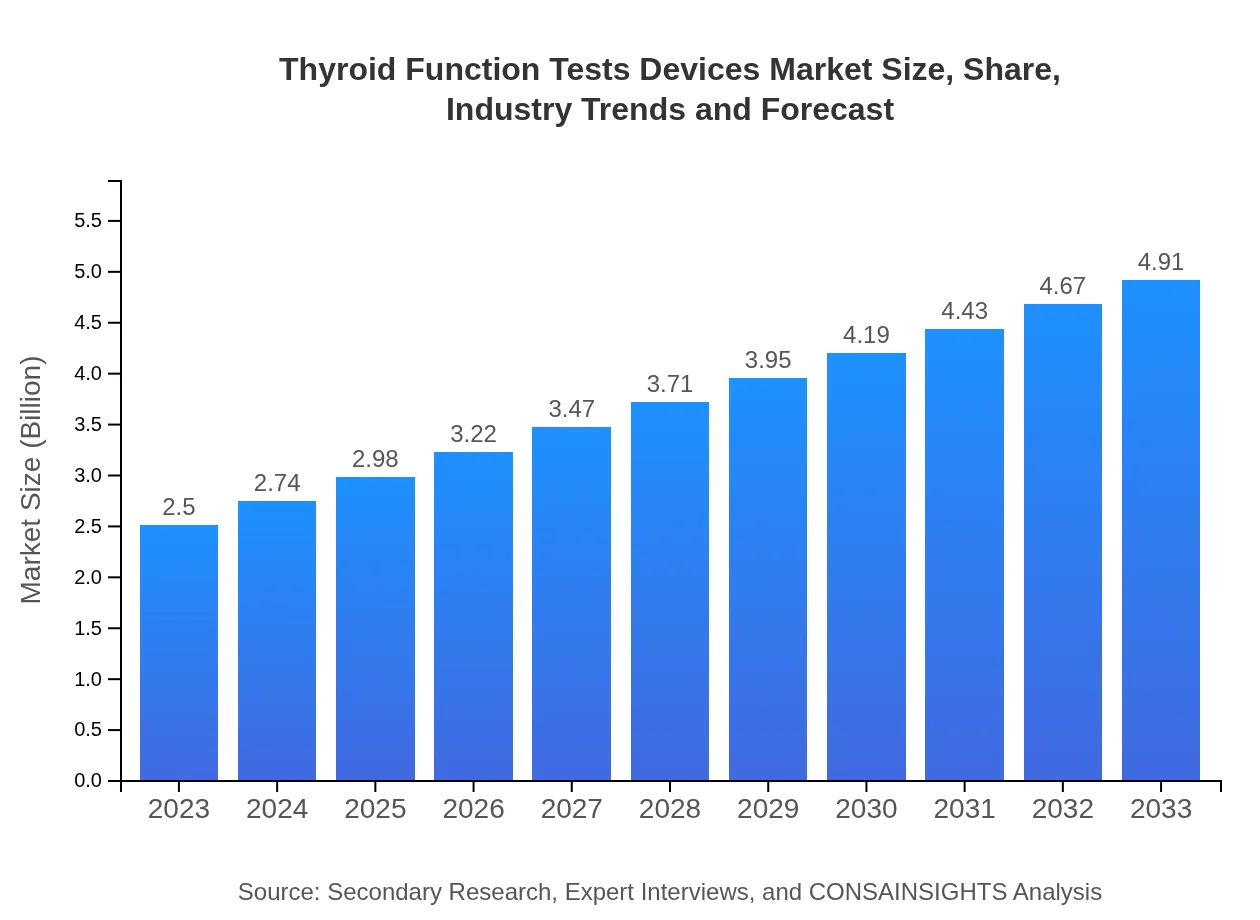
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, AbbVie Inc. |
| Last Modified Date | 31 January 2026 |
Thyroid Function Tests Devices Market Overview
Customize Thyroid Function Tests Devices Market Report market research report
- ✔ Get in-depth analysis of Thyroid Function Tests Devices market size, growth, and forecasts.
- ✔ Understand Thyroid Function Tests Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Thyroid Function Tests Devices
What is the Market Size & CAGR of Thyroid Function Tests Devices market in 2023?
Thyroid Function Tests Devices Industry Analysis
Thyroid Function Tests Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Thyroid Function Tests Devices Market Analysis Report by Region
Europe Thyroid Function Tests Devices Market Report:
In Europe, the Thyroid Function Tests Devices market is expected to experience considerable growth from $0.82 billion in 2023 to $1.61 billion by 2033. Increased investment in R&D, along with higher awareness about thyroid-related health issues, are key growth drivers. Enhanced relationships between healthcare institutions and manufacturers also promote faster adoption of innovative testing devices.Asia Pacific Thyroid Function Tests Devices Market Report:
The Asia Pacific region is emerging as a significant market for Thyroid Function Tests Devices, with a projected market size of $0.78 billion by 2033, up from $0.40 billion in 2023. Increasing healthcare expenditure, coupled with a rising prevalence of thyroid disorders, is driving this growth. Furthermore, advancements in medical technology and growing awareness are anticipated to boost the adoption of these tests in countries like China and India.North America Thyroid Function Tests Devices Market Report:
North America holds a significant share of the Thyroid Function Tests Devices market, with a size of $0.92 billion in 2023, anticipated to reach $1.80 billion by 2033. The rise in thyroid disorders, along with technological advancements, supports this growth. The presence of major market players and emphasis on preventive healthcare further enhance market dynamics in the region.South America Thyroid Function Tests Devices Market Report:
In South America, the market for Thyroid Function Tests Devices is expected to grow from $0.10 billion in 2023 to $0.21 billion by 2033. Factors such as increasing healthcare access and growing initiatives from various health organizations are contributing to the market's expansion in this region. However, the market may face challenges due to economic fluctuations and healthcare disparities.Middle East & Africa Thyroid Function Tests Devices Market Report:
The Middle East and Africa region is projected to see growth from $0.27 billion in 2023 to $0.52 billion by 2033 in the Thyroid Function Tests Devices market. Despite challenges such as limited healthcare infrastructure in some countries, rising investment in healthcare improvements is expected to create growth opportunities, particularly in urban areas.Tell us your focus area and get a customized research report.
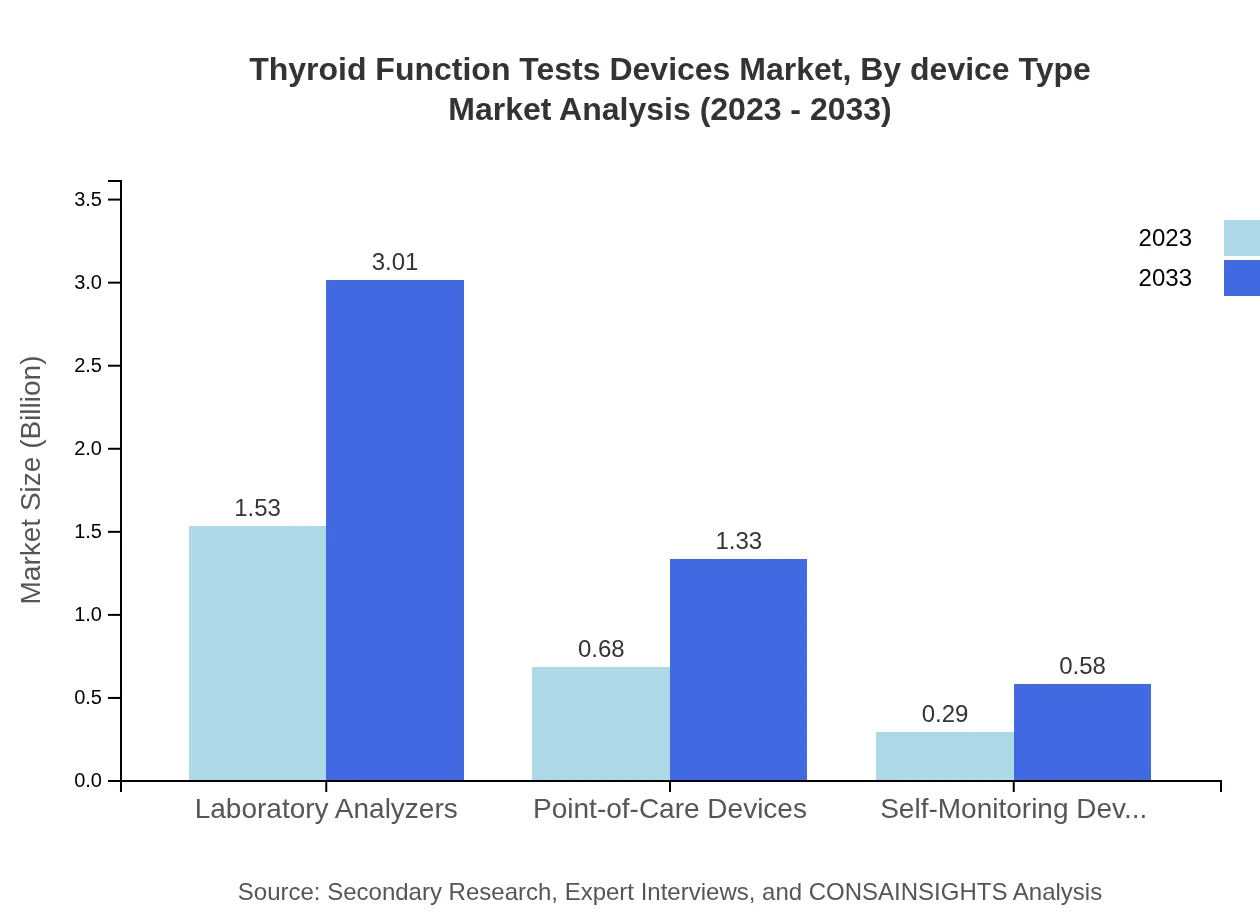
Thyroid Function Tests Devices Market Analysis By Device Type
The Thyroid Function Tests Devices market is segmented by device type, where laboratory analyzers dominate with a projected market size of $3.01 billion by 2033, demonstrating significant advances in accuracy and efficiency for hospital settings. Point-of-care devices are also gaining traction due to their convenience and rapid results, expected to grow to $1.33 billion in the same timeframe.
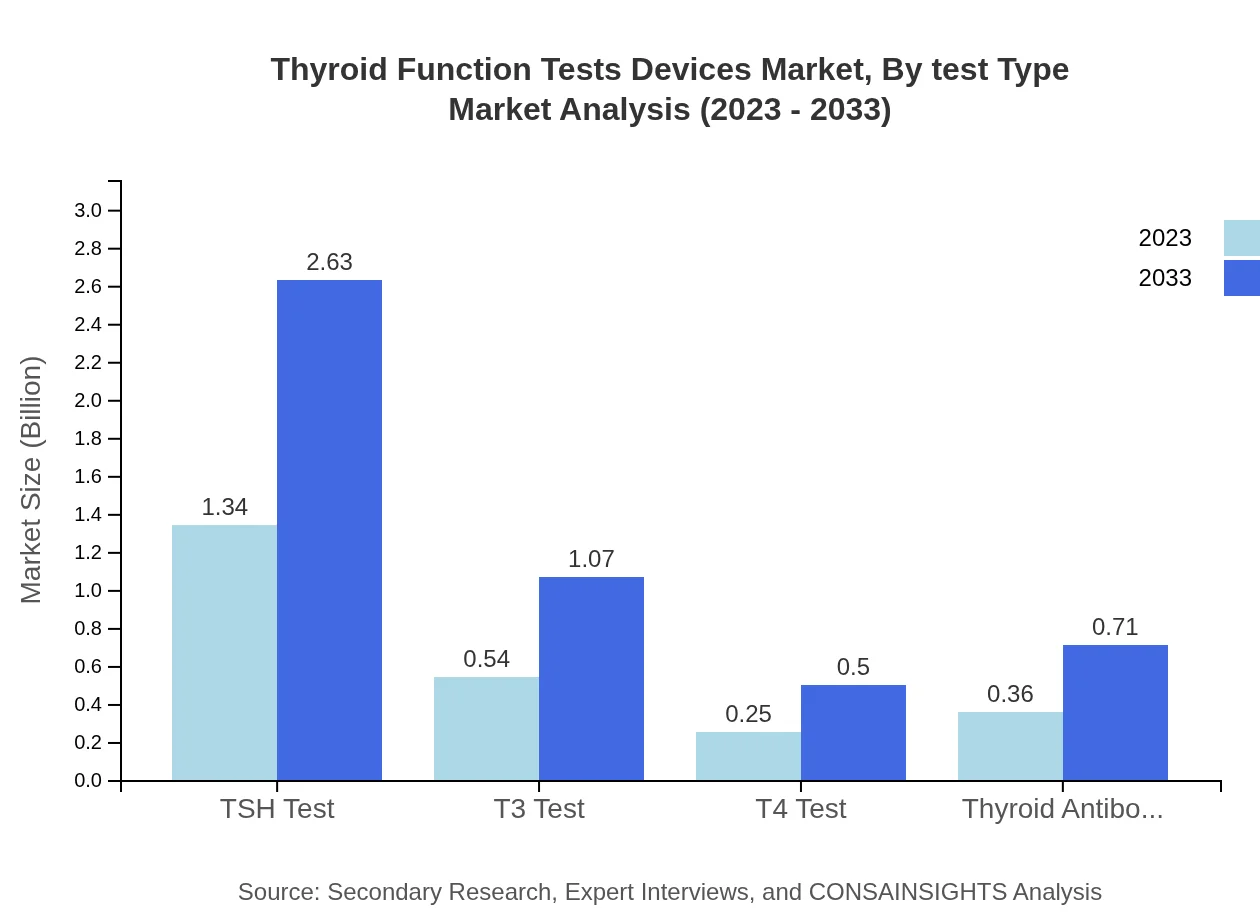
Thyroid Function Tests Devices Market Analysis By Test Type
In the test type segmentation, TSH tests account for the largest market share, expected to maintain this position with a projected value of $2.63 billion by 2033. T3 and T4 tests, while smaller in share, are anticipated to grow in tandem with increasing diagnosis rates and public awareness, expected to reach $1.07 billion and $0.50 billion respectively.
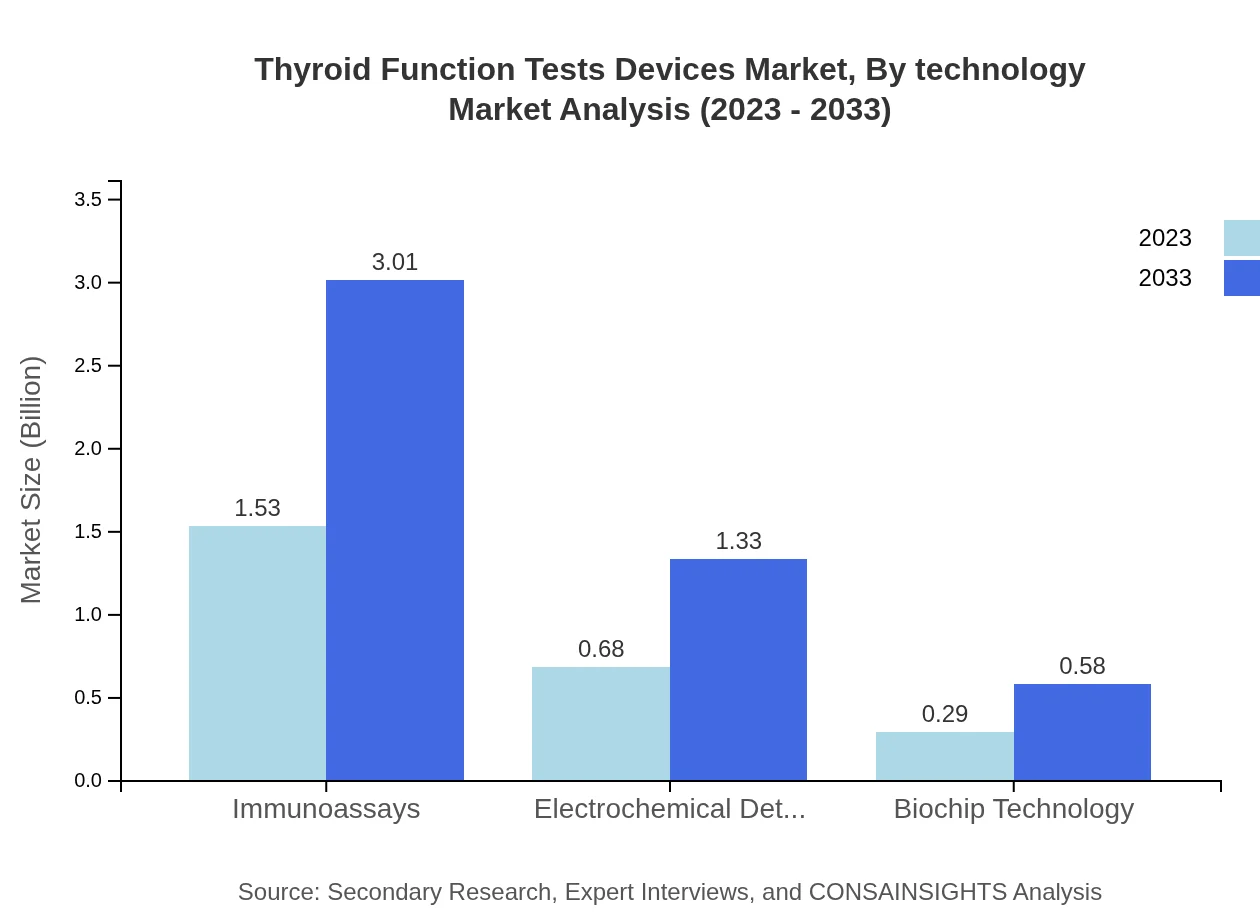
Thyroid Function Tests Devices Market Analysis By Technology
From a technology perspective, immunoassays lead the Thyroid Function Tests market with a significant forecast growth to $3.01 billion by 2033, due to their high accuracy and reliability. Electrochemical detection and biochip technology also show promising growth rates, focusing on increasing efficiency and faster analysis times.
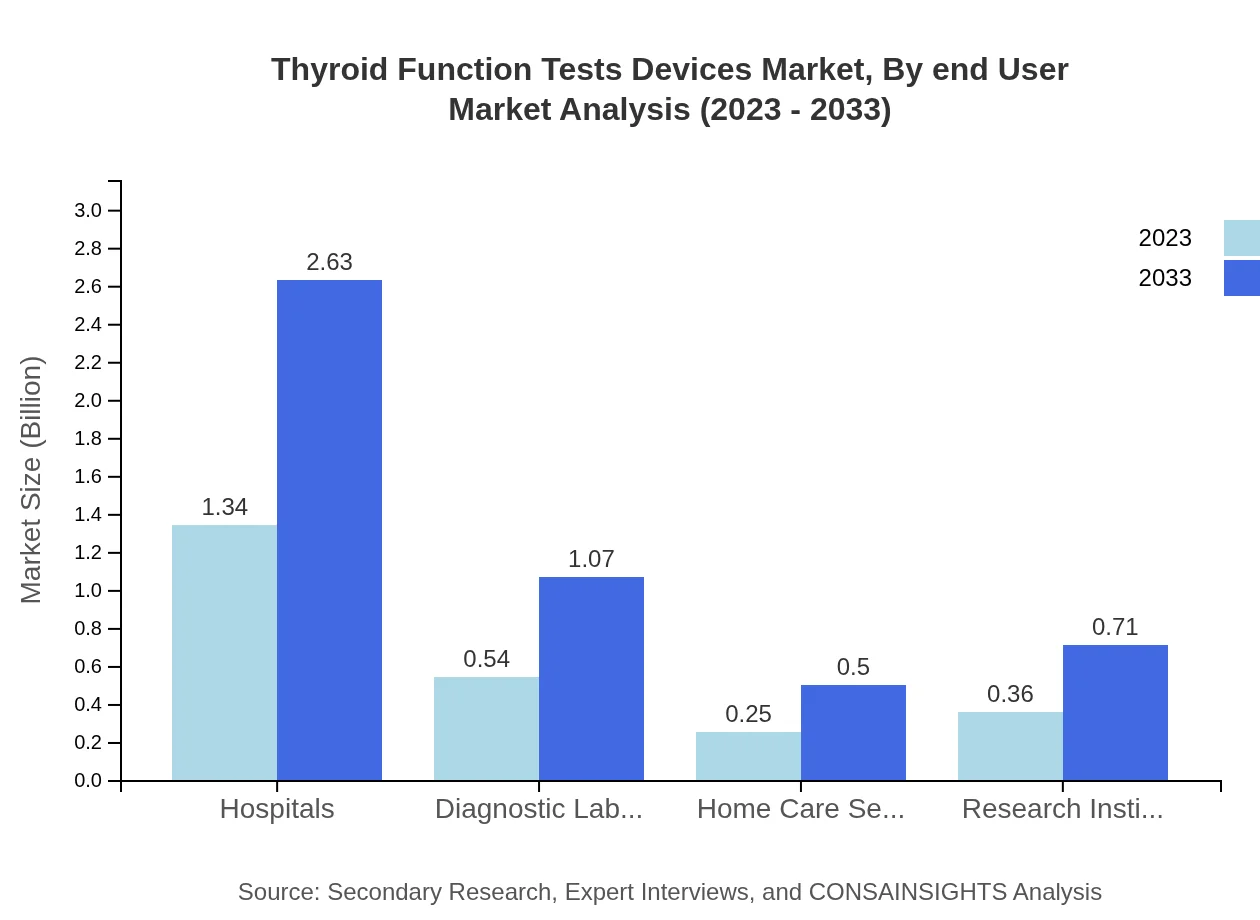
Thyroid Function Tests Devices Market Analysis By End User
Hospitals will remain the leading end-user segment, projecting to maintain a larger share at 53.59% and an estimated size of $2.63 billion by 2033. Diagnostic laboratories will also grow significantly, expected to reach $1.07 billion, while home care settings and research institutes will contribute to market expansion through innovative testing solutions.
Thyroid Function Tests Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Thyroid Function Tests Devices Industry
Abbott Laboratories:
A global healthcare leader, Abbott Laboratories specializes in developing advanced diagnostics, including thyroid function tests, enhancing patient care through innovative technologies.Roche Diagnostics:
Roche Diagnostics is a pioneer in laboratory diagnostics, providing comprehensive testing solutions for thyroid function assessment, focusing on accuracy and efficiency in medical testing.Siemens Healthineers:
Siemens Healthineers offers cutting-edge diagnostic solutions, emphasizing the development of precise testing devices for thyroid function, contributing to better health outcomes.Thermo Fisher Scientific:
Thermo Fisher Scientific is known for its innovation in laboratory products and services, including a wide range of thyroid testing solutions designed for both research and clinical applications.AbbVie Inc.:
AbbVie Inc. focuses on pharmaceutical and diagnostic products, actively contributing to advancements in thyroid health diagnostics through continuous research and innovative development.We're grateful to work with incredible clients.









FAQs
What is the market size of thyroid Function Tests Devices?
The global market size for thyroid function tests devices is estimated at 2.5 billion in 2023, with a projected CAGR of 6.8% from 2023 to 2033, indicating robust growth potential in the coming years.
What are the key market players or companies in this thyroid Function Tests Devices industry?
Key players in the thyroid function tests devices market include Abbott Laboratories, Siemens Healthineers, Roche Diagnostics, and Thermo Fisher Scientific, known for their extensive portfolios in diagnostic technologies, contributing significantly to market advancements.
What are the primary factors driving the growth in the thyroid function tests devices industry?
The growth in the thyroid function tests devices market is driven by an increasing prevalence of thyroid disorders, rising health awareness, advancements in diagnostic technologies, and the growing demand for early disease detection across healthcare settings.
Which region is the fastest Growing in the thyroid Function Tests Devices?
In the thyroid function tests devices market, Europe is the fastest-growing region, projected to grow from $0.82 billion in 2023 to $1.61 billion by 2033, reflecting significant healthcare investments and advancements in diagnostic solutions.
Does ConsaInsights provide customized market report data for the thyroid Function Tests Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the thyroid function tests devices industry, allowing clients to gain precise insights based on their individual requirements and market dynamics.
What deliverables can I expect from this thyroid Function Tests Devices market research project?
From the thyroid function tests devices market research project, clients can expect comprehensive reports, data analysis, market forecasts, regional insights, competitor assessments, and actionable recommendations to support strategic decisions.
What are the market trends of thyroid Function Tests Devices?
Current trends in the thyroid function tests devices market include technological innovations, the rise of point-of-care testing, increased adoption of self-monitoring devices, and a growing focus on home care settings for chronic disease management.