Tourniquets Device Market Report
Published Date: 31 January 2026 | Report Code: tourniquets-device
Tourniquets Device Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive analysis of the Tourniquets Device market, including insights on market size, growth potential, segmentation, regional analysis, and future trends from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
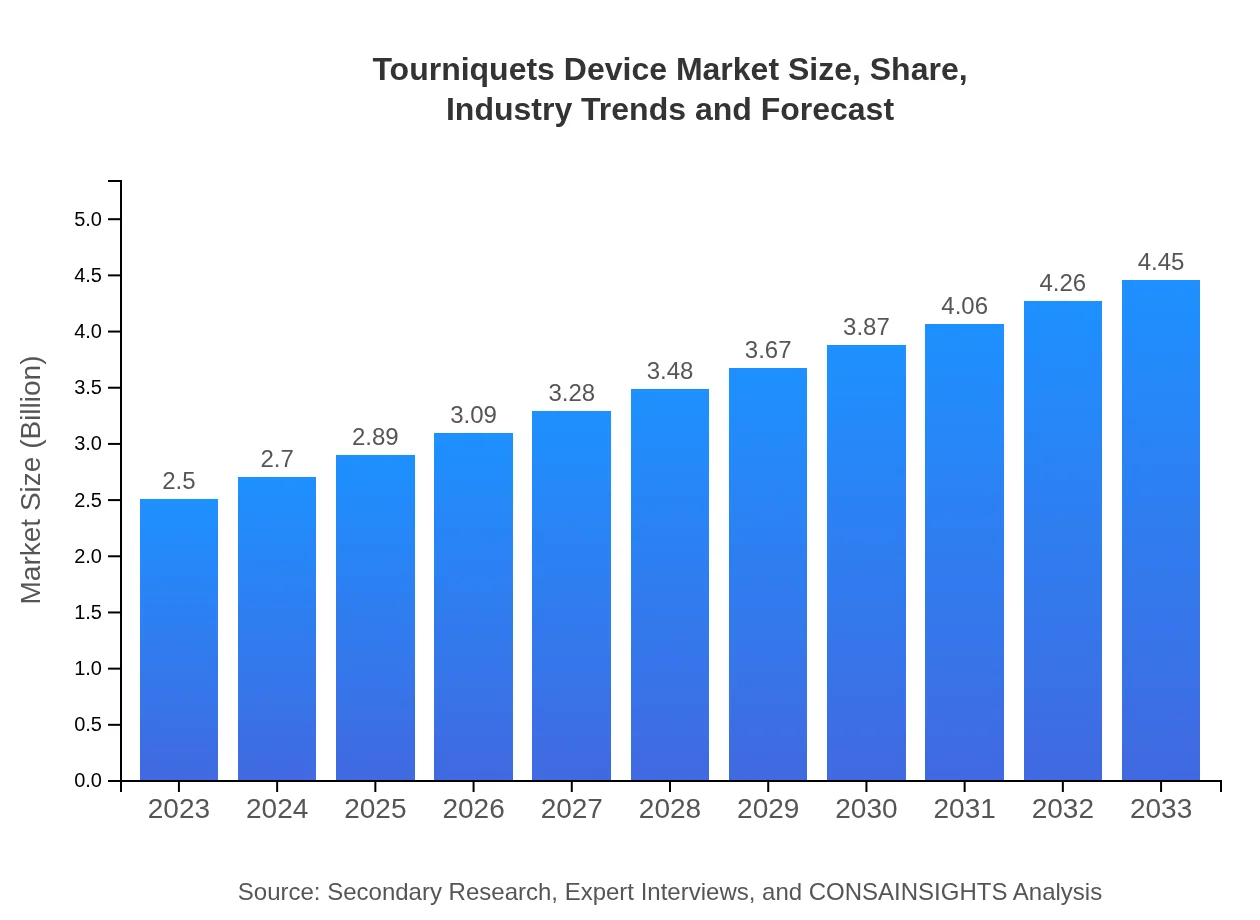
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $4.45 Billion |
| Top Companies | Medline Industries, Inc., Stryker Corporation, Z-Medica, LLC, SAM Medical Products |
| Last Modified Date | 31 January 2026 |
Tourniquets Device Market Overview
Customize Tourniquets Device Market Report market research report
- ✔ Get in-depth analysis of Tourniquets Device market size, growth, and forecasts.
- ✔ Understand Tourniquets Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Tourniquets Device
What is the Market Size & CAGR of Tourniquets Device market in 2023?
Tourniquets Device Industry Analysis
Tourniquets Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Tourniquets Device Market Analysis Report by Region
Europe Tourniquets Device Market Report:
The European market is forecasted to grow from $0.68 billion in 2023 to $1.22 billion by 2033. Increasing incidences of strokes and cardiovascular diseases, coupled with a robust emergency medical service sector, are the primary factors contributing to this growth.Asia Pacific Tourniquets Device Market Report:
The Asia Pacific region is expected to witness substantial growth, with the market reaching approximately $0.97 billion by 2033, up from $0.55 billion in 2023. Rapid urbanization, increased healthcare expenditure, and a growing population are driving demand for advanced medical devices in this region.North America Tourniquets Device Market Report:
North America is currently the largest market, with a value of $0.82 billion in 2023 projected to rise to $1.46 billion by 2033. The presence of major healthcare institutions, high demand for advanced medical products, and ongoing research funding are key growth drivers.South America Tourniquets Device Market Report:
In South America, the market for Tourniquets Devices is forecasted to grow from $0.25 billion in 2023 to $0.44 billion by 2033. Improvements in healthcare infrastructure and an emphasis on trauma care are expected to bolster market growth.Middle East & Africa Tourniquets Device Market Report:
In the Middle East and Africa, the market is expected to grow from $0.20 billion in 2023 to $0.36 billion by 2033. The increasing need for emergency medical services in conflict zones and a rising awareness of advanced trauma care fundamentally support market expansion in this region.Tell us your focus area and get a customized research report.
Tourniquets Device Market Analysis By Type
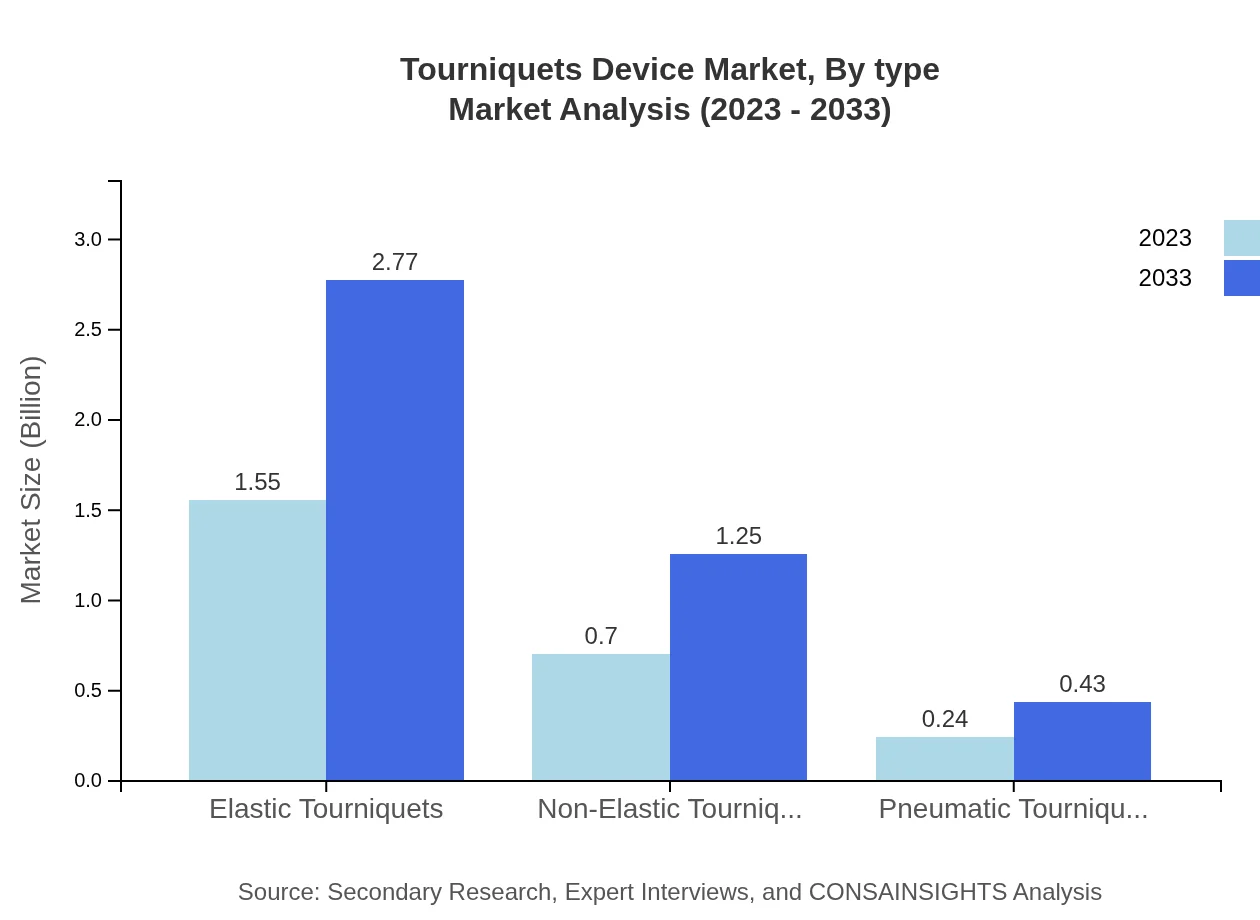
The market consists primarily of elastic and non-elastic tourniquets, with elastic tourniquets holding the majority market share. Elastic Tourniquets are projected to grow from $1.55 billion in 2023 to $2.77 billion in 2033, while non-elastic types are expected to reach $1.25 billion. Additionally, pneumatic tourniquets will see incremental growth, forecasted at $0.43 billion by 2033.
Tourniquets Device Market Analysis By Application
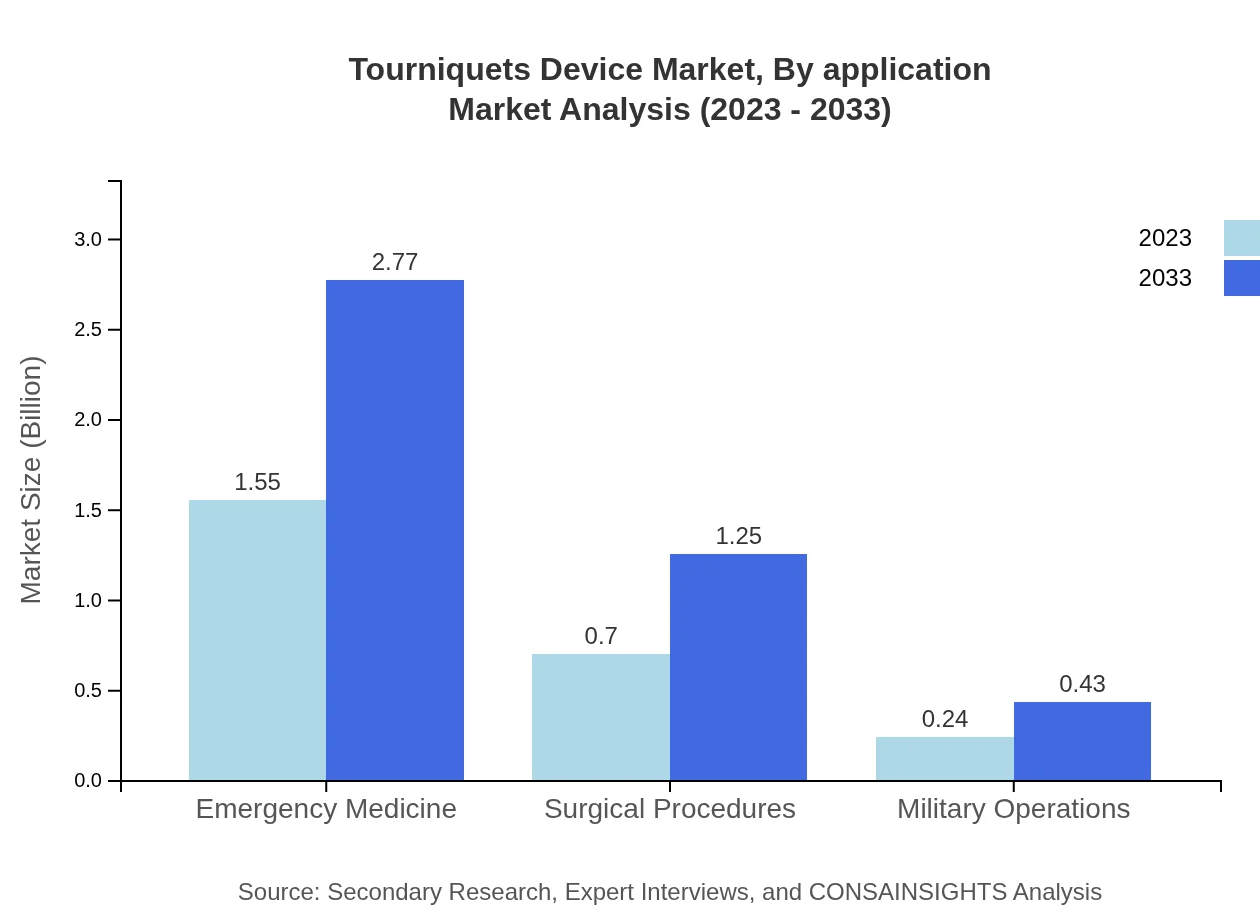
Applications are segmented into emergency medicine, surgical procedures, military operations, and at-home care. Emergency medicine currently dominates the market, expected to grow from $1.55 billion to $2.77 billion by 2033, reflecting the rise of trauma cases and the need for immediate bleeding control.
Tourniquets Device Market Analysis By End User
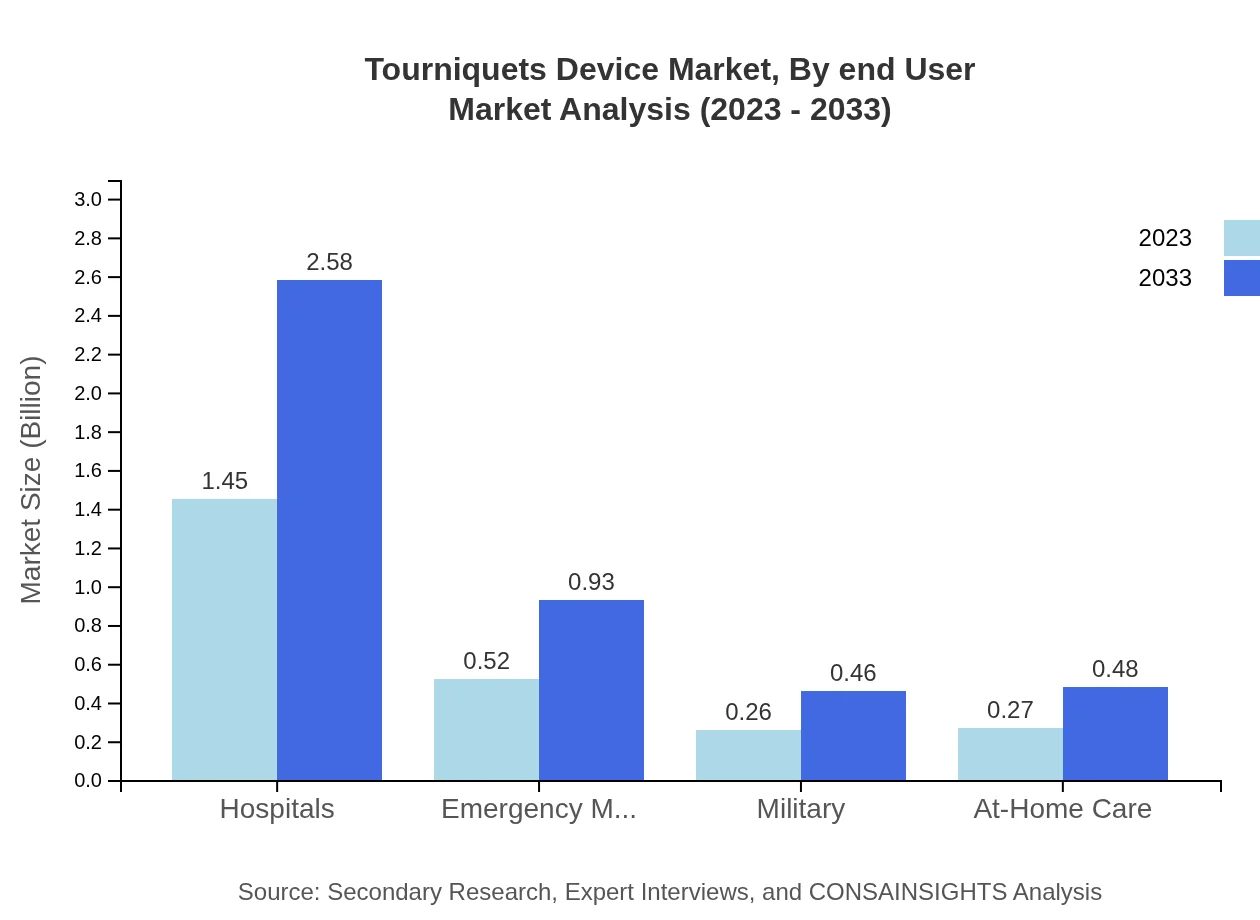
End-users are categorized into hospitals, emergency medical services, military, and at-home care. Hospitals represent the largest share, growing from $1.45 billion in 2023 to $2.58 billion by 2033, highlighting the critical role of tourniquets in hospital settings and emergency response scenarios.
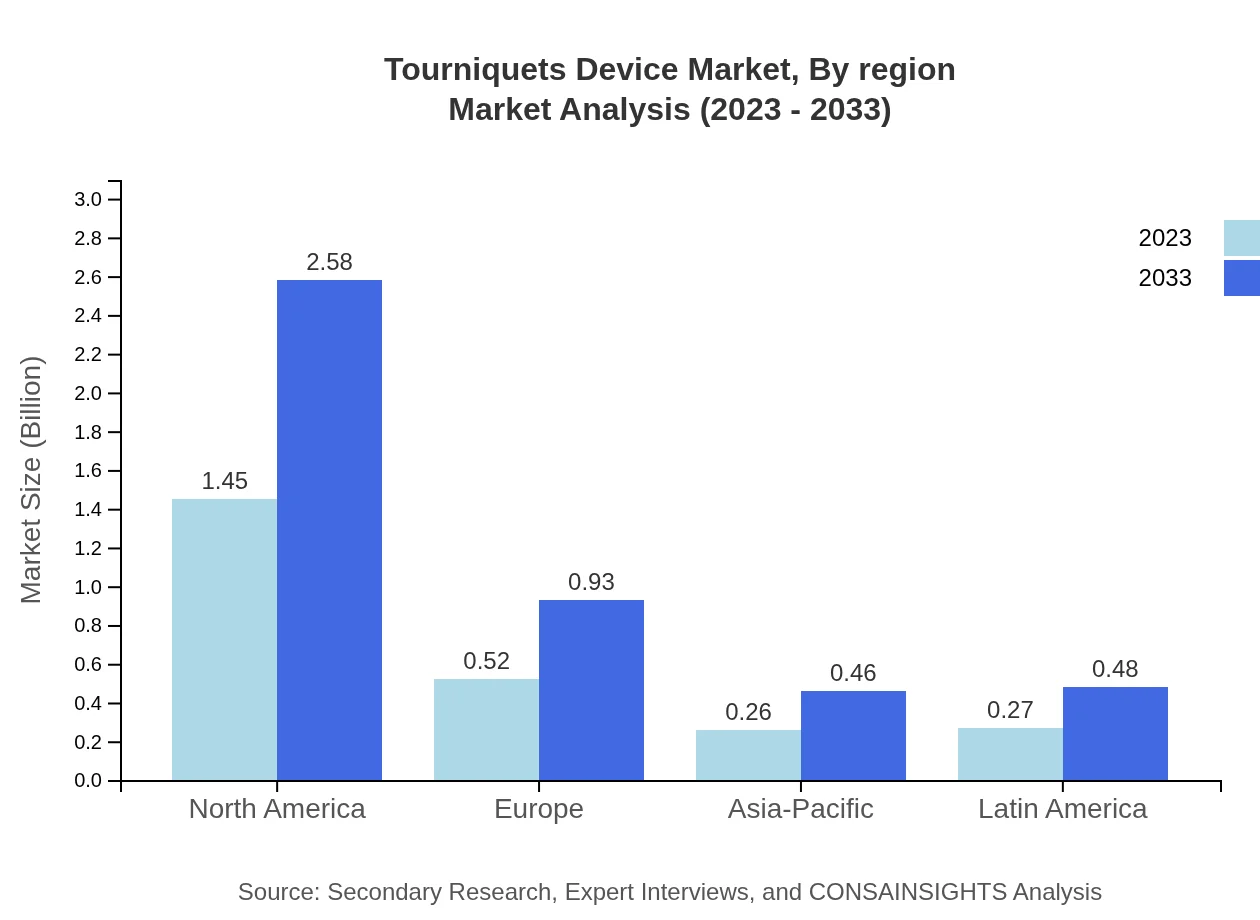
Tourniquets Device Market Analysis By Region
Regionally, the market analysis indicates North America as the current leader, followed by Europe and the Asia Pacific. Each region presents unique opportunities based on local healthcare needs, regulatory environments, and trauma care strategies influencing demand for tourniquets.
Tourniquets Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Tourniquets Device Industry
Medline Industries, Inc.:
A leading manufacturer of medical supplies, Medline offers a range of tourniquets designed for emergency and hospital use, focusing on quality and compliance with safety standards.Stryker Corporation:
Known for its advanced medical technologies, Stryker produces a range of innovative tourniquet systems designed for trauma situations, ensuring rapid response capabilities.Z-Medica, LLC:
Specializing in hemorrhage control solutions, Z-Medica provides innovative tourniquet products that are widely used in military and civilian emergency medicine.SAM Medical Products:
SAM Medical focuses on developing advanced trauma products, including the SAM Junctional Tourniquet, which has gained popularity for its effectiveness in controlling severe bleeding.We're grateful to work with incredible clients.









FAQs
What is the market size of tourniquets Device?
As of 2023, the tourniquet device market is valued at approximately $2.5 billion, with a projected CAGR of 5.8% from 2023 to 2033, highlighting significant growth dynamics over the next decade.
What are the key market players or companies in this tourniquets Device industry?
Key players in the tourniquets-device market include established medical technology companies with specialty offerings in emergency medicine and surgical equipment. These players continuously innovate to meet evolving healthcare demands.
What are the primary factors driving the growth in the tourniquets Device industry?
The growth in the tourniquets-device industry is propelled by rising incidences of trauma cases, advancements in medical technology, and increased awareness of emergency medical care standards across healthcare facilities globally.
Which region is the fastest Growing in the tourniquets Device market?
North America is the fastest-growing region in the tourniquet device market, projected to grow from $0.82 billion in 2023 to $1.46 billion by 2033, reflecting an increasing focus on emergency medical services.
Does ConsaInsights provide customized market report data for the tourniquets Device industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the tourniquets-device industry, allowing stakeholders to obtain relevant insights for informed strategic decisions.
What deliverables can I expect from this tourniquets Device market research project?
Deliverables for the tourniquets-device market research project typically include comprehensive reports, detailed market analyses, regional insights, and trend forecasts, facilitating a well-rounded understanding of this dynamic market.
What are the market trends of tourniquets Device?
Current trends in the tourniquets-device market include a shift towards the development of advanced materials and designs, increasing automation in emergency response equipment, and a growing emphasis on training for healthcare professionals.