Transcatheter Mitral Valve Repair Replacement Market Report
Published Date: 31 January 2026 | Report Code: transcatheter-mitral-valve-repair-replacement
Transcatheter Mitral Valve Repair Replacement Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Transcatheter Mitral Valve Repair Replacement market, focusing on current trends, segmentation, regional analysis, technologies, and forecasts for the period 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
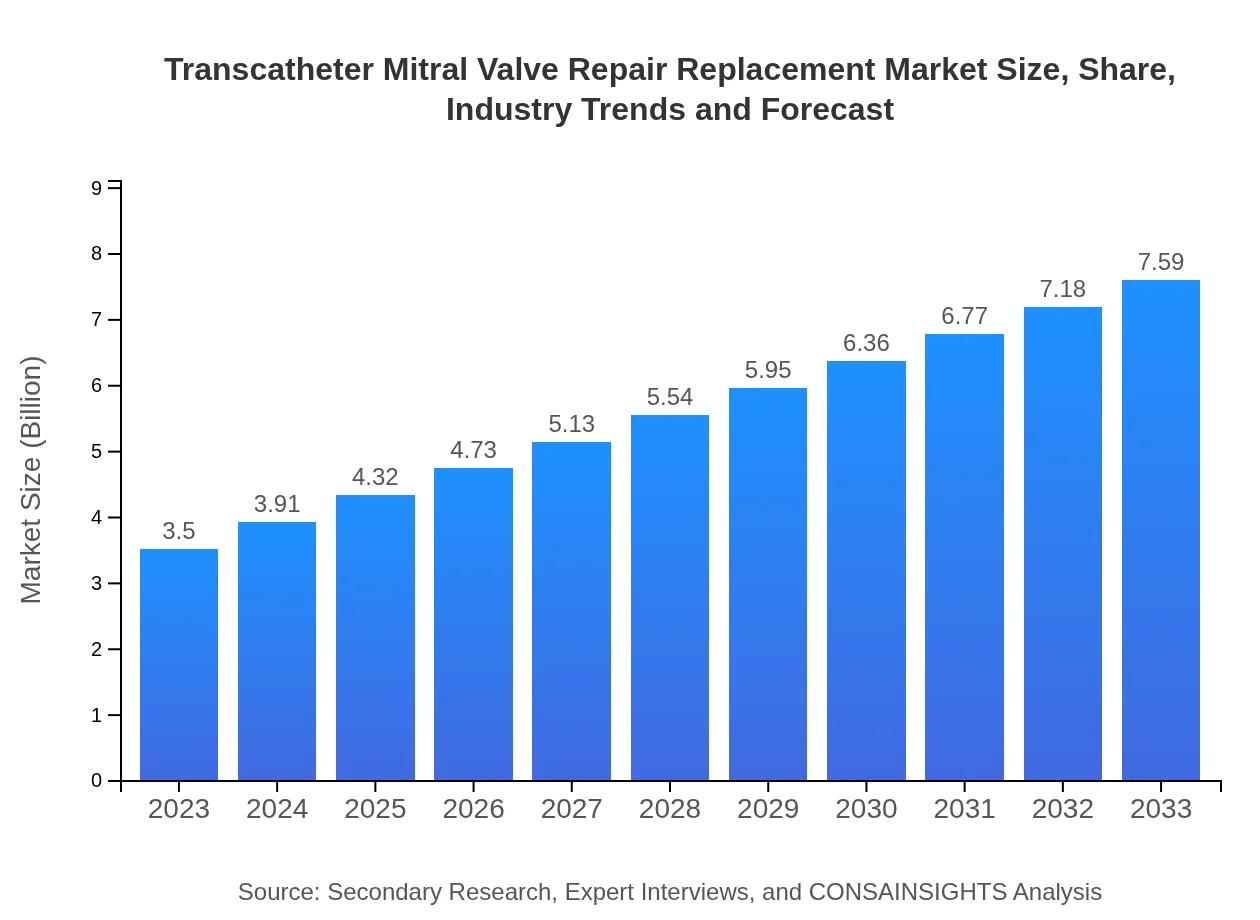
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $7.59 Billion |
| Top Companies | Edwards Lifesciences, Medtronic , Abbott Laboratories, Boston Scientific |
| Last Modified Date | 31 January 2026 |
Transcatheter Mitral Valve Repair Replacement Market Overview
Customize Transcatheter Mitral Valve Repair Replacement Market Report market research report
- ✔ Get in-depth analysis of Transcatheter Mitral Valve Repair Replacement market size, growth, and forecasts.
- ✔ Understand Transcatheter Mitral Valve Repair Replacement's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Transcatheter Mitral Valve Repair Replacement
What is the Market Size & CAGR of Transcatheter Mitral Valve Repair Replacement market in 2023?
Transcatheter Mitral Valve Repair Replacement Industry Analysis
Transcatheter Mitral Valve Repair Replacement Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Transcatheter Mitral Valve Repair Replacement Market Analysis Report by Region
Europe Transcatheter Mitral Valve Repair Replacement Market Report:
Europe's market is projected to grow from $0.89 billion in 2023 to $1.93 billion by 2033. A strong focus on research, development, and healthcare innovation drives market expansion, complemented by increasing patient population seeking innovative treatment options.Asia Pacific Transcatheter Mitral Valve Repair Replacement Market Report:
The Asia Pacific region is experiencing significant market growth, projected to increase from $0.74 billion in 2023 to $1.60 billion by 2033. Rising healthcare investments, an increasing elderly population, and the adoption of advanced medical technologies are driving factors in this region.North America Transcatheter Mitral Valve Repair Replacement Market Report:
North America leads the market, with growth from $1.28 billion in 2023 to $2.78 billion in 2033. High healthcare expenditure, advanced treatment facilities, and a favorable regulatory environment contribute to this robust growth.South America Transcatheter Mitral Valve Repair Replacement Market Report:
In South America, the market size is expected to rise from $0.25 billion in 2023 to $0.54 billion by 2033. Challenges in healthcare access and economic fluctuations are mitigated by growing awareness and government initiatives to improve cardiac care.Middle East & Africa Transcatheter Mitral Valve Repair Replacement Market Report:
The Middle East and Africa market is expected to expand from $0.34 billion in 2023 to $0.73 billion by 2033, fueled by improving healthcare infrastructure and rising awareness about cardiovascular diseases.Tell us your focus area and get a customized research report.
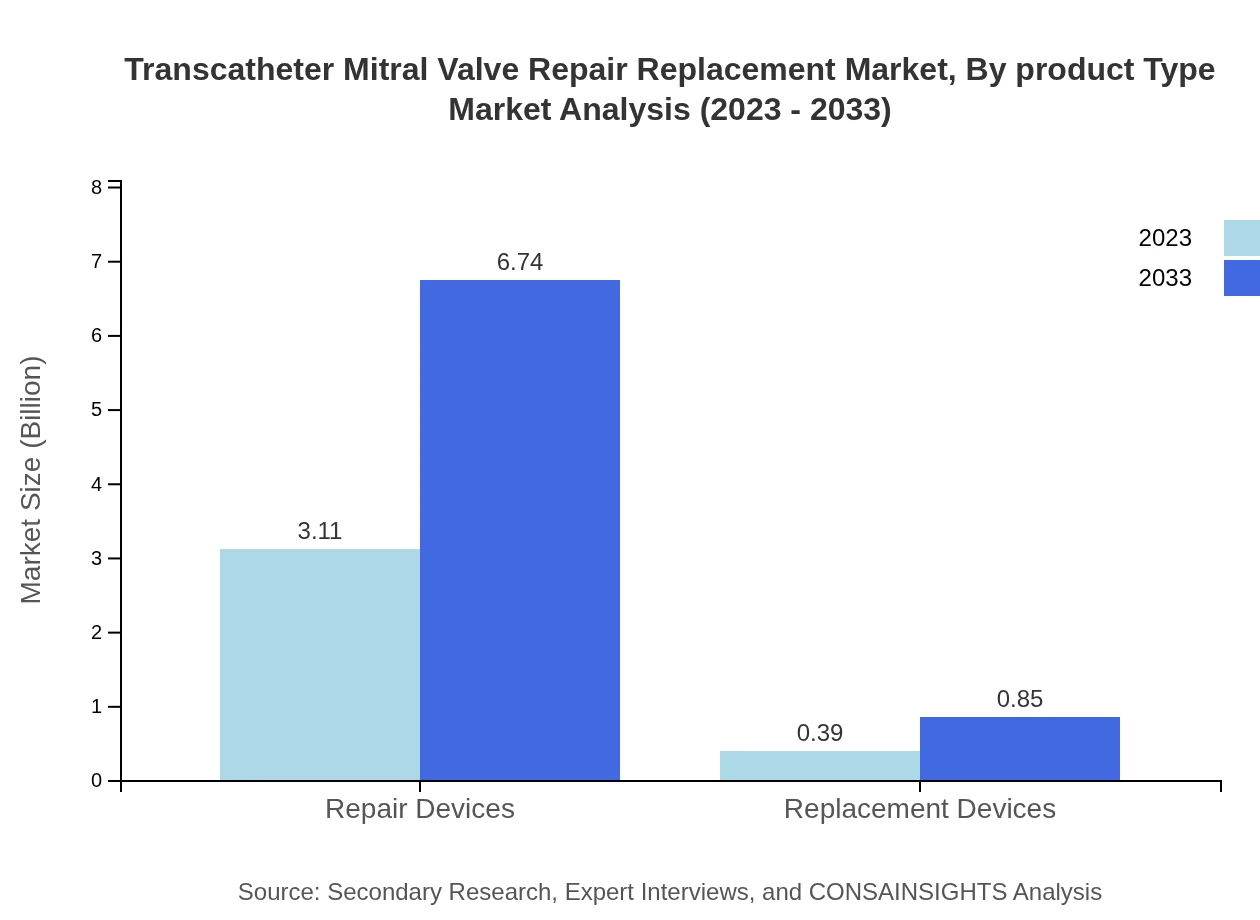
Transcatheter Mitral Valve Repair Replacement Market Analysis By Product Type
The market is primarily differentiated into Repair Devices and Replacement Devices. Repair Devices constitute the substantial segment with a market size of $3.11 billion, gaining 88.79% market share in 2023. The primary reason for this dominance is the preference for repair approaches that tend to have fewer complications. Replacement Devices account for $0.39 billion, representing an 11.21% share, appealing mainly in specific cases where repair is not viable.
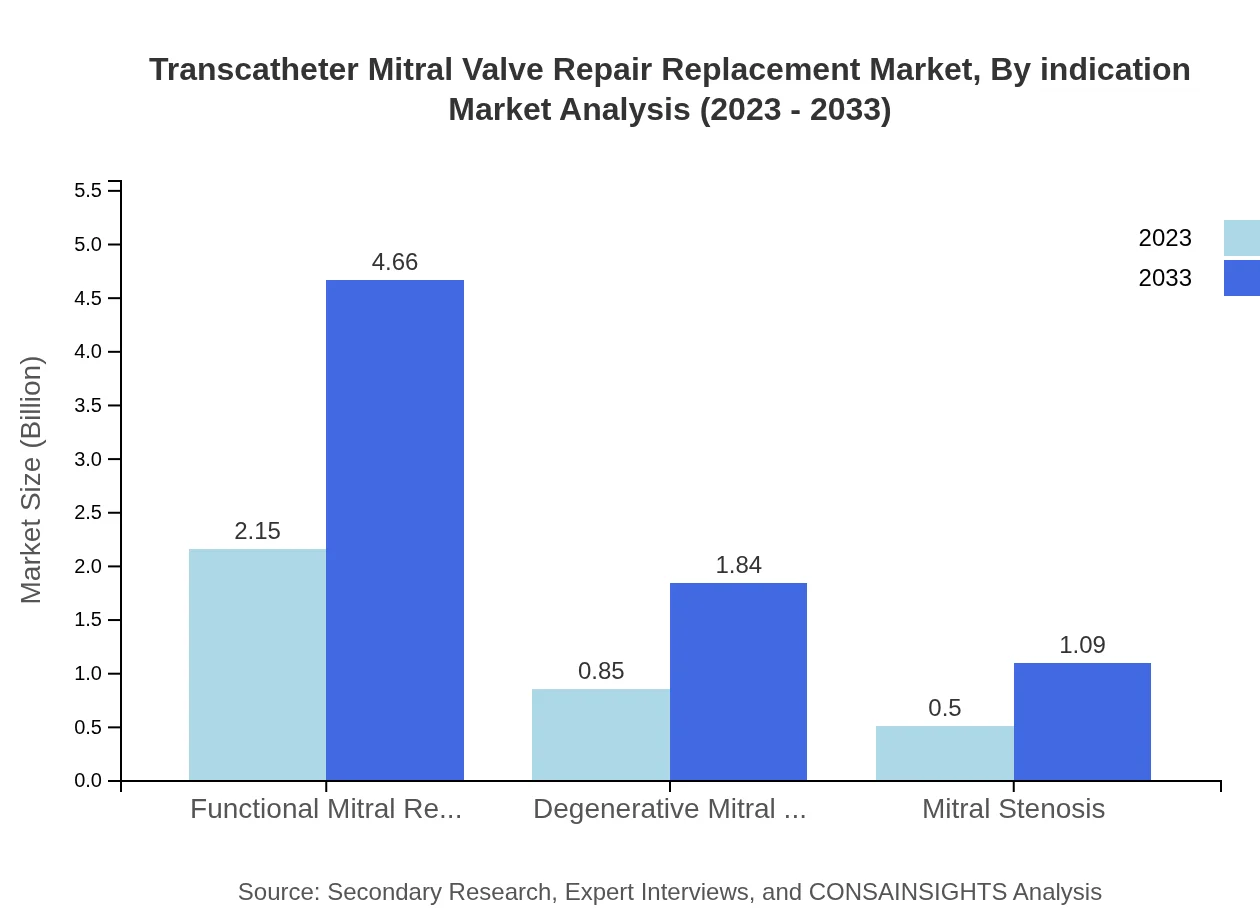
Transcatheter Mitral Valve Repair Replacement Market Analysis By Indication
By indication, the market is categorized into Functional Mitral Regurgitation, Degenerative Mitral Regurgitation, and Mitral Stenosis. Functional Mitral Regurgitation leads the segment with a projected market share of 61.37%, attributed to the higher prevalence of functional disorders. Degenerative cases are also prominent, holding 24.28% market share, while Mitral Stenosis represents 14.35%, signaling a lesser incidence of this condition.
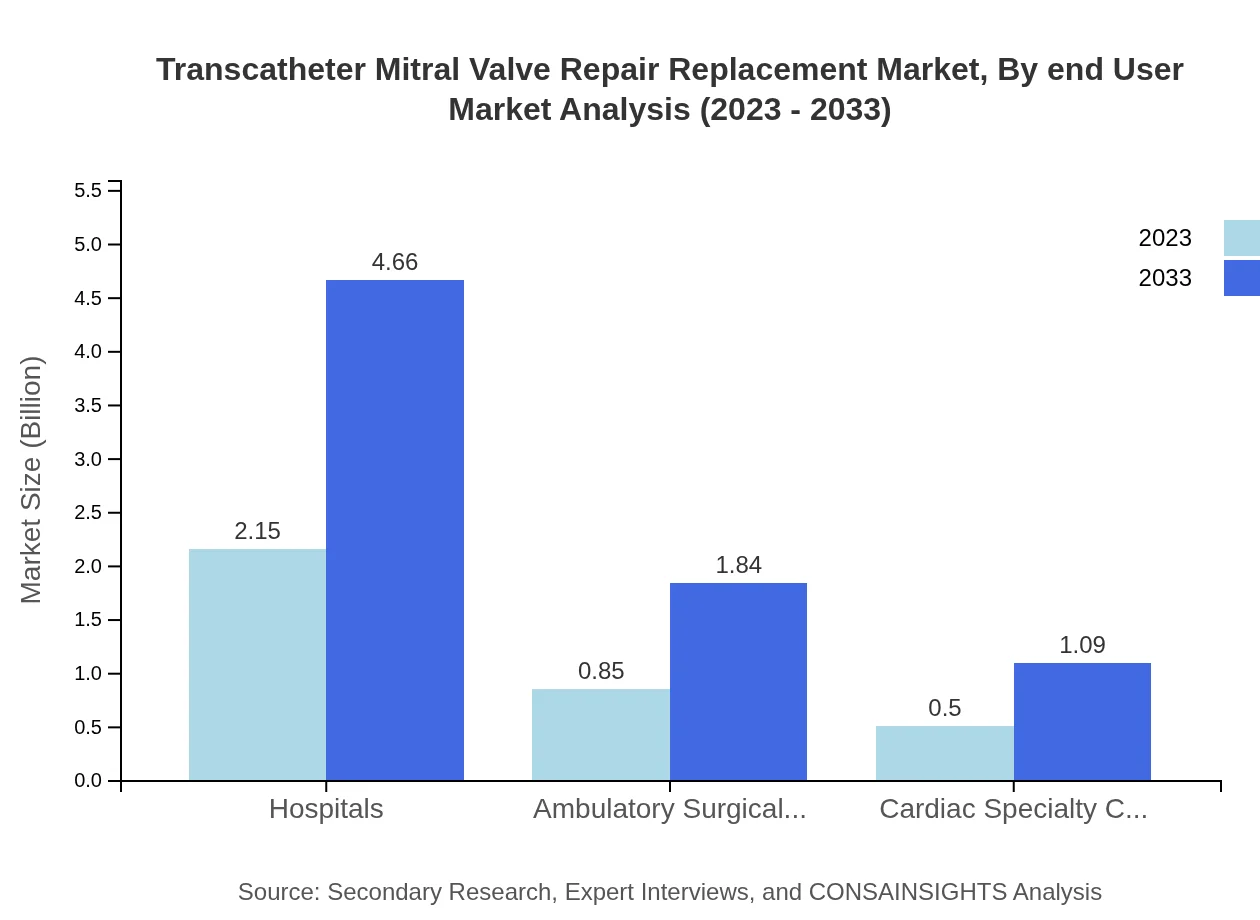
Transcatheter Mitral Valve Repair Replacement Market Analysis By End User
Hospitals emerge as the primary end-user segment, commanding a substantial 61.37% market share in 2023, indicative of their advanced resources and patient turnover. Ambulatory Surgical Centers, with a share of 24.28%, are gaining traction due to the shift towards outpatient procedures. Cardiac Specialty Clinics hold 14.35%, focusing more on customized care for heart patients, thereby expanding their influence in the segment.
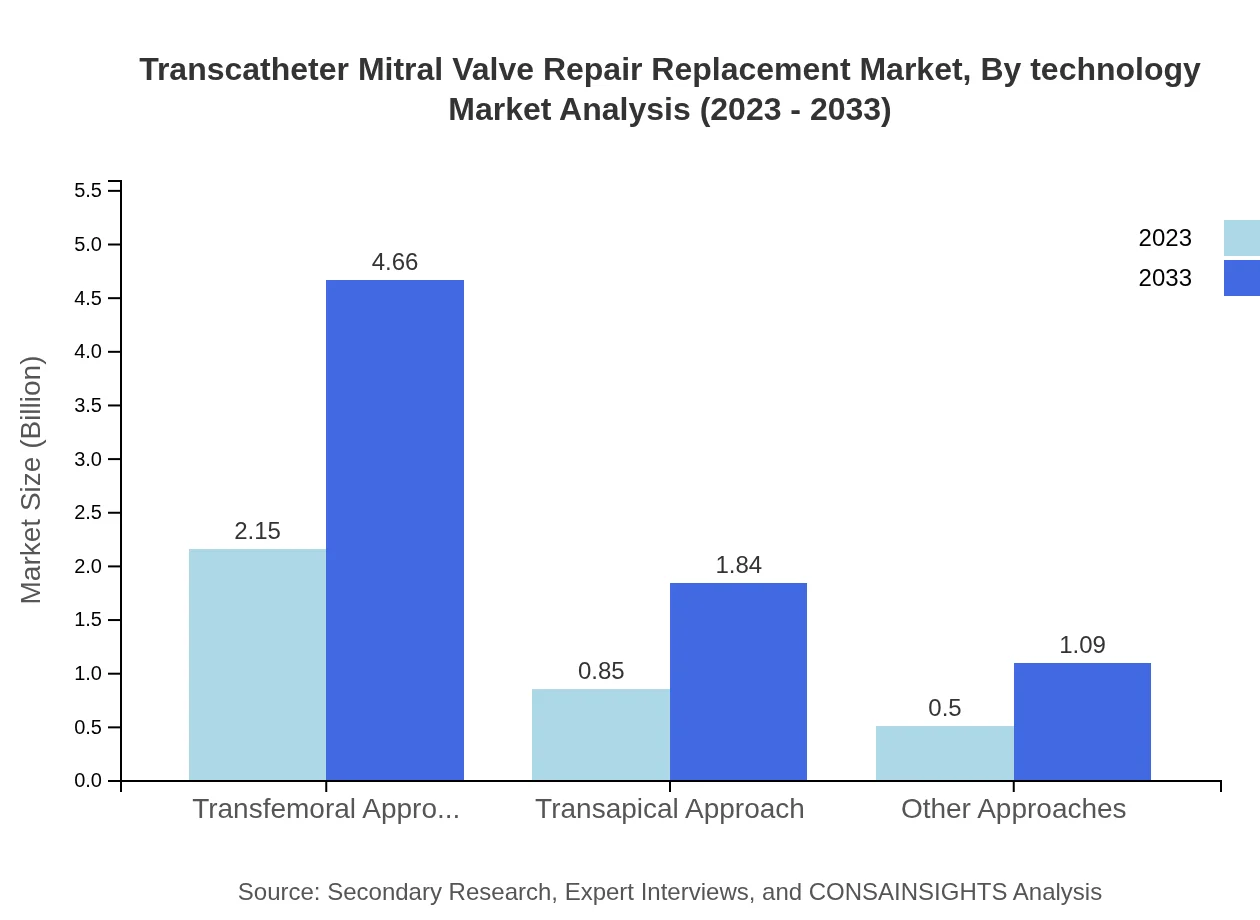
Transcatheter Mitral Valve Repair Replacement Market Analysis By Technology
The market is driven predominantly by the Transfemoral Approach, which holds 61.37% market share due to its minimally invasive nature and efficacy. The Transapical Approach is also significant, accounting for 24.28%, while other approaches represent 14.35%, indicating ongoing innovation and adaptability in procedural techniques.
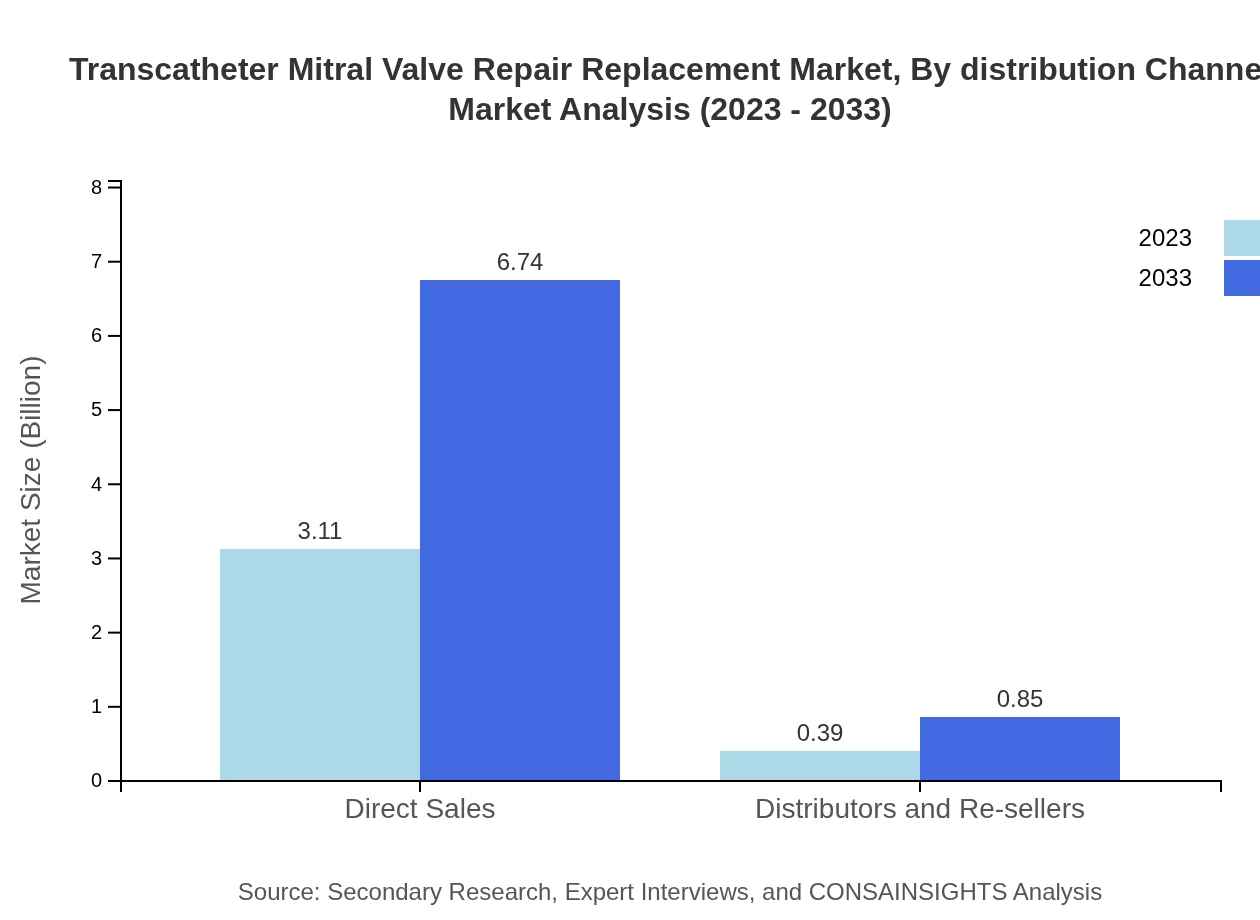
Transcatheter Mitral Valve Repair Replacement Market Analysis By Distribution Channel
The distribution channels are mainly dominated by Direct Sales, accounting for 88.79% of the market share due to the direct relationship with healthcare providers. Distributors and re-sellers hold 11.21%, reflecting an established channel for reaching a broader audience while supporting market expansion.
Transcatheter Mitral Valve Repair Replacement Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Transcatheter Mitral Valve Repair Replacement Industry
Edwards Lifesciences:
A prominent player in the field, Edwards Lifesciences specializes in heart valves, including innovative transcatheter technologies for mitral valve replacements.Medtronic :
Medtronic is known for its significant contributions to cardiovascular therapy, offering advanced solutions for mitral valve repair and replacement.Abbott Laboratories:
Abbott is a critical innovator in the cardiovascular space, focusing on minimally invasive procedures to address mitral valve insufficiencies.Boston Scientific:
Boston Scientific is recognized for its comprehensive portfolio of devices that enhance the treatment of various cardiac conditions, including mitral valve disorders.We're grateful to work with incredible clients.









FAQs
What is the market size of transcatheter mitral valve repair replacement?
The global market for transcatheter mitral valve repair replacement is valued at $3.5 billion in 2023, with a projected CAGR of 7.8% from 2023 to 2033, indicating robust growth in this medical sector.
What are the key market players or companies in this transcatheter mitral valve repair replacement industry?
Key market players in the transcatheter mitral valve repair replacement industry include Medtronic, Abbott Laboratories, Boston Scientific, Edwards Lifesciences, and other leading medical device manufacturers focusing on cardiac solutions and innovations.
What are the primary factors driving the growth in the transcatheter mitral valve repair replacement industry?
The growth of the transcatheter mitral valve repair replacement market is driven by increasing incidences of mitral valve disorders, advancements in minimally invasive procedures, and enhanced patient outcomes associated with transcatheter techniques.
Which region is the fastest Growing in the transcatheter mitral valve repair replacement?
The Asia Pacific region is the fastest-growing market for transcatheter mitral valve repair replacement, projected to grow from $0.74 billion in 2023 to $1.60 billion by 2033, reflecting significant demand and healthcare investment.
Does ConsaInsights provide customized market report data for the transcatheter mitral valve repair replacement industry?
Yes, ConsaInsights offers customized market report data tailored specifically for the transcatheter mitral valve repair replacement industry, providing detailed insights and analyses to meet unique client needs.
What deliverables can I expect from this transcatheter mitral valve repair replacement market research project?
Deliverables from the transcatheter mitral valve repair replacement market research project include comprehensive market analysis reports, regional evaluations, competitive landscape assessments, and future trend forecasts.
What are the market trends of transcatheter mitral valve repair replacement?
Current market trends in transcatheter mitral valve repair replacement include the rise of novel repair techniques, advancements in device technology, increasing regulatory approvals, and a growing shift towards outpatient care settings.