Transcatheter Pulmonary Valve Market Report
Published Date: 31 January 2026 | Report Code: transcatheter-pulmonary-valve
Transcatheter Pulmonary Valve Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Transcatheter Pulmonary Valve market, covering market overview, segmentation, regional insights, and trends through 2033. It aims to offer valuable data and insights for stakeholders in the healthcare industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
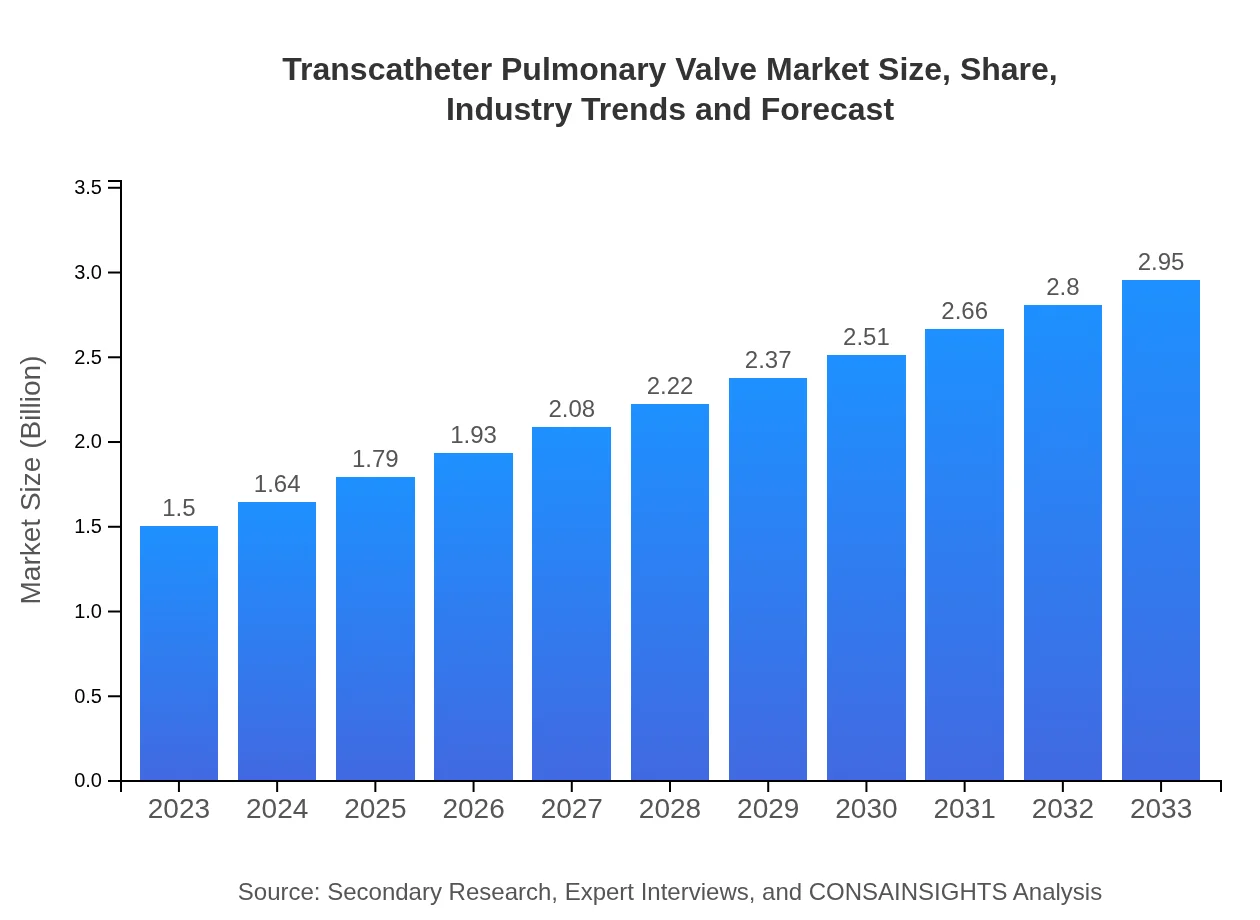
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | Edwards Lifesciences Corporation, Medtronic Plc, St. Jude Medical, Boston Scientific Corporation |
| Last Modified Date | 31 January 2026 |
Transcatheter Pulmonary Valve Market Overview
Customize Transcatheter Pulmonary Valve Market Report market research report
- ✔ Get in-depth analysis of Transcatheter Pulmonary Valve market size, growth, and forecasts.
- ✔ Understand Transcatheter Pulmonary Valve's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Transcatheter Pulmonary Valve
What is the Market Size & CAGR of Transcatheter Pulmonary Valve market in 2023?
Transcatheter Pulmonary Valve Industry Analysis
Transcatheter Pulmonary Valve Market Segmentation and Scope
Tell us your focus area and get a customized research report.
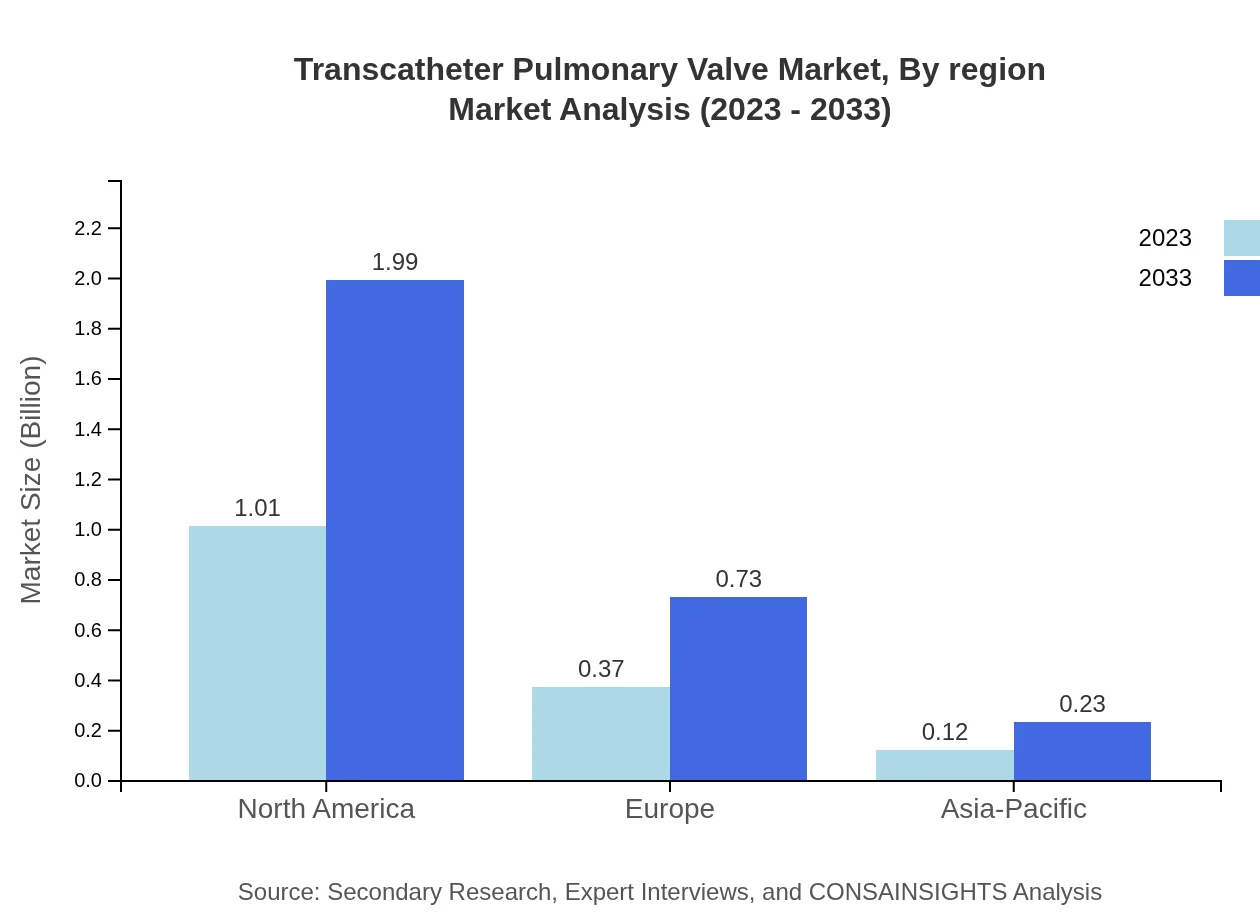
Transcatheter Pulmonary Valve Market Analysis Report by Region
Europe Transcatheter Pulmonary Valve Market Report:
The European market for Transcatheter Pulmonary Valves is forecasted to increase from USD 0.37 billion in 2023 to USD 0.72 billion by 2033, driven by favorable regulatory environments and increasing prevalence of heart valve disorders.Asia Pacific Transcatheter Pulmonary Valve Market Report:
The Asia-Pacific region is witnessing steady growth in the TPV market, projected to reach USD 0.57 billion by 2033 from USD 0.29 billion in 2023. Factors such as rising healthcare expenditure and increasing awareness of advanced cardiovascular treatments drive this expansion.North America Transcatheter Pulmonary Valve Market Report:
North America holds a significant share of the TPV market, with a value projected to grow from USD 0.54 billion in 2023 to USD 1.07 billion by 2033. Factors include robust technological advancements, high adoption rates, and the presence of key market players.South America Transcatheter Pulmonary Valve Market Report:
In South America, the TPV market is expected to grow from USD 0.11 billion in 2023 to USD 0.22 billion by 2033, attributed to improving healthcare infrastructure and rising incidences of congenital heart diseases.Middle East & Africa Transcatheter Pulmonary Valve Market Report:
Market growth in the Middle East and Africa is anticipated to rise from USD 0.19 billion in 2023 to USD 0.37 billion by 2033, fueled by improvements in healthcare access and a growing focus on cardiac health.Tell us your focus area and get a customized research report.
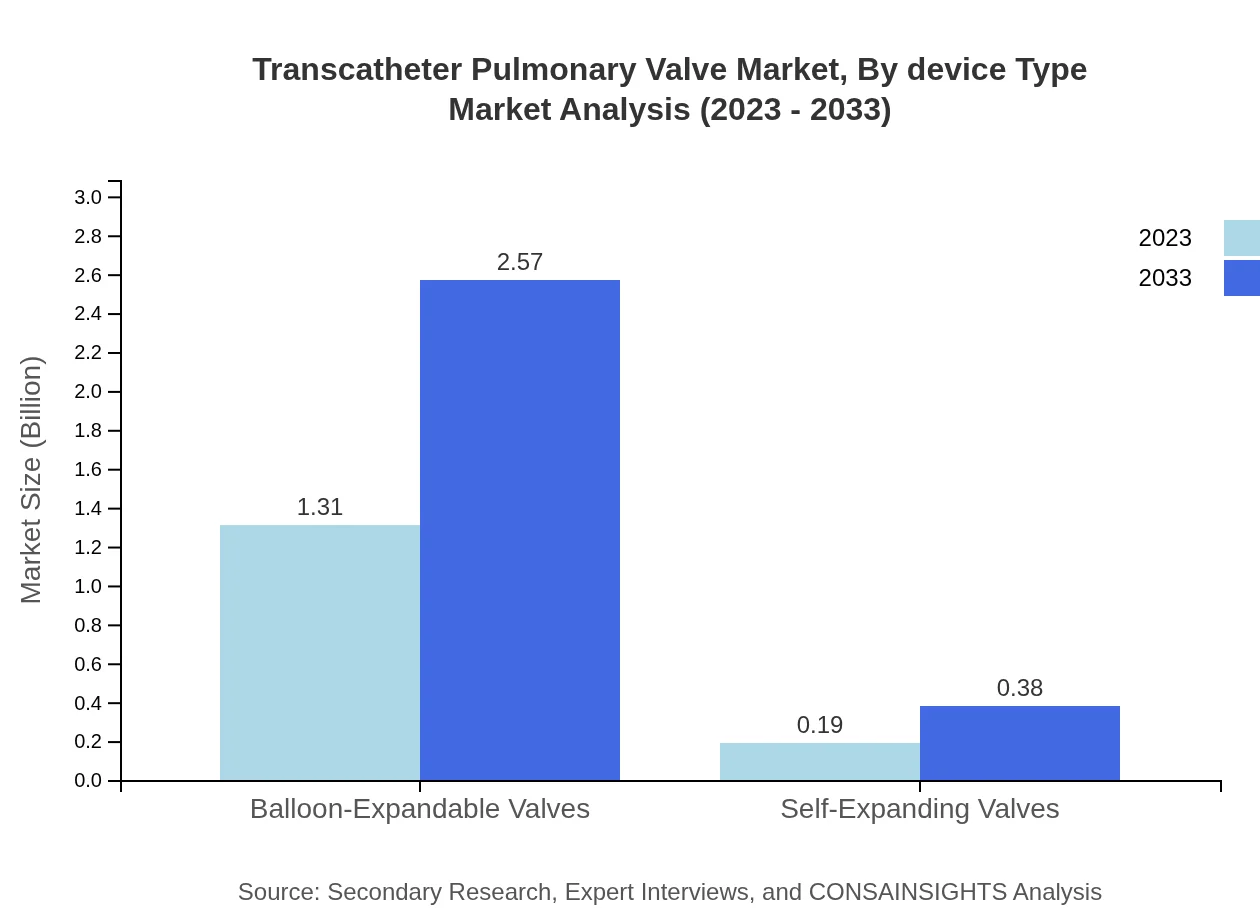
Transcatheter Pulmonary Valve Market Analysis By Device Type
The TPV market by device type is primarily divided into two segments: balloon-expandable valves and self-expanding valves. Balloon-expandable valves are estimated to account for the majority of the market due to their established efficacy and ease of use, while self-expanding valves are gaining popularity for their adaptability to patient anatomy.
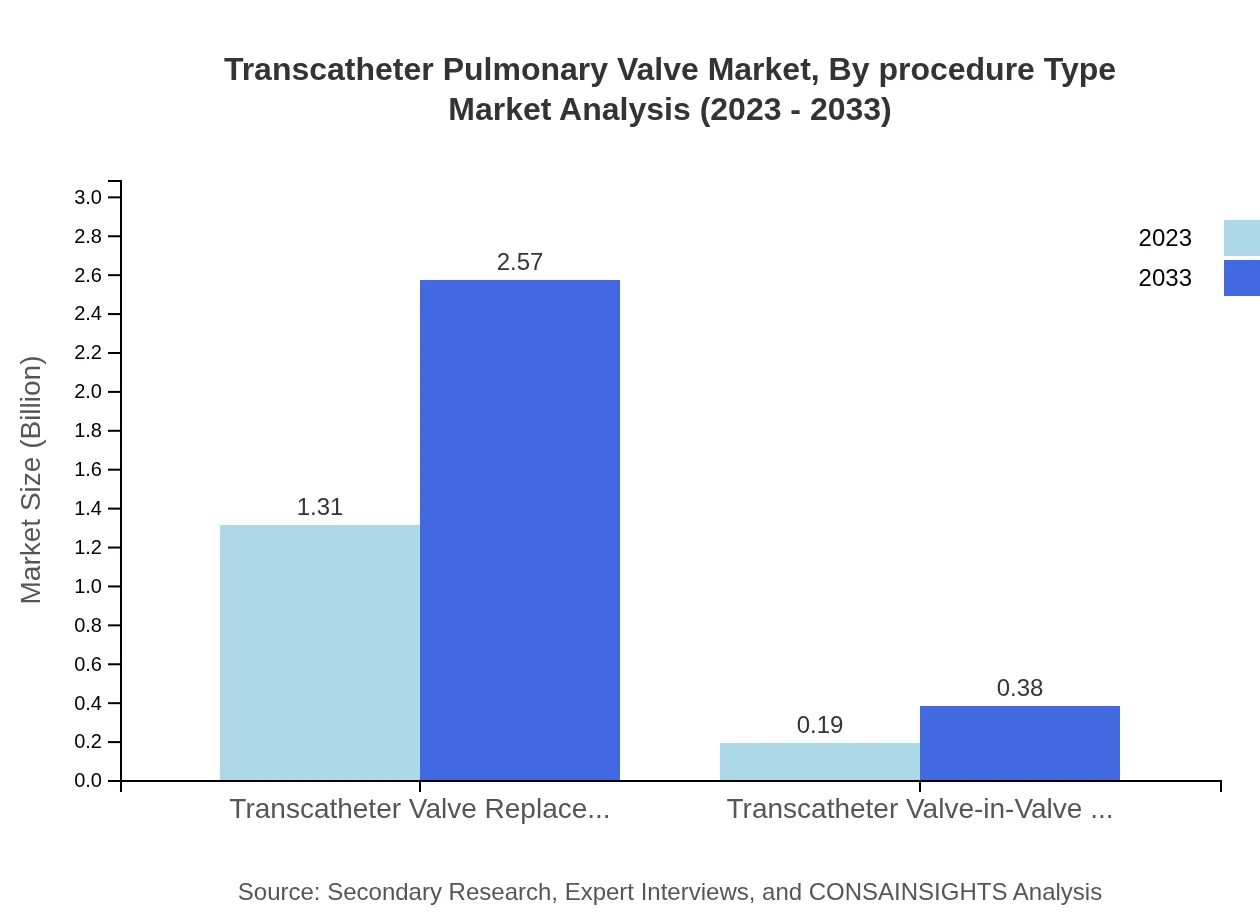
Transcatheter Pulmonary Valve Market Analysis By Procedure Type
By procedure type, the market is segmented into transcatheter valve replacement and valve-in-valve repairs. Transcatheter valve replacement is the dominant segment due to the rise in patients opting for minimally invasive solutions, whereas valve-in-valve procedures are crucial for treating patients with existing valve prosthetics.
Transcatheter Pulmonary Valve Market Analysis By End User
The market by end-user is segmented into hospitals and ambulatory surgical centers. Hospitals are the leading segment, accounting for a significant share of the market, as they offer comprehensive surgical care and advanced facilities for managing complex cases.
Transcatheter Pulmonary Valve Market Analysis By Region
The regional analysis indicates that North America will continue to lead the market, closely followed by Europe and Asia-Pacific. North America will command the largest market share due to its advanced healthcare system and established market players.
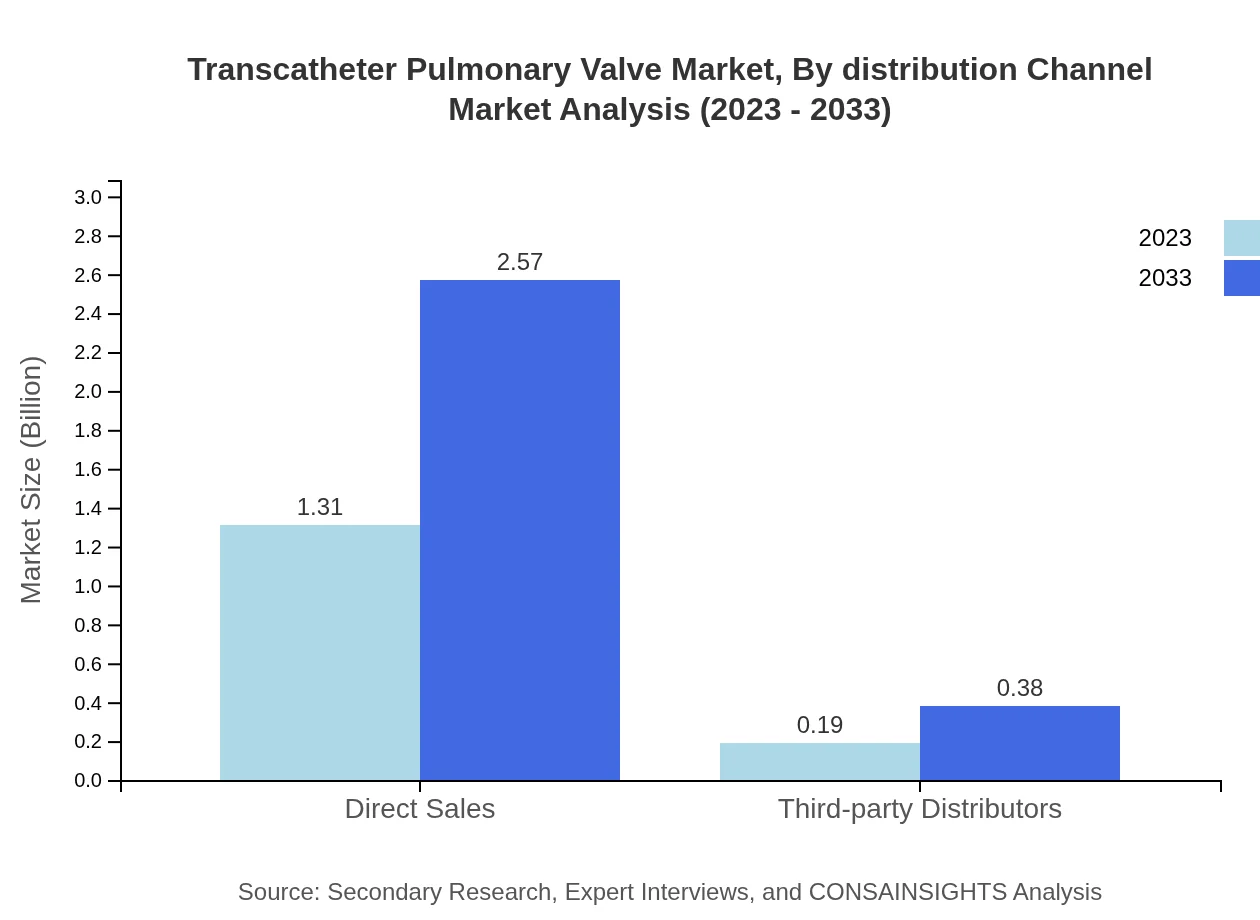
Transcatheter Pulmonary Valve Market Analysis By Distribution Channel
In terms of distribution channels, direct sales constitute the major share, owing to manufacturers’ strategies to establish strong distributor networks. However, third-party distributors are steadily gaining ground as healthcare systems seek diverse sourcing options.
Transcatheter Pulmonary Valve Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Transcatheter Pulmonary Valve Industry
Edwards Lifesciences Corporation:
A leading company in heart valve therapies, known for its innovative products like the SAPIEN valve, which has revolutionized the treatment of aortic stenosis and is expanding into TPV.Medtronic Plc:
A major player in the medical device market, Medtronic is recognized for its comprehensive portfolio of cardiac devices, including transcatheter valves, contributing significantly to the TPV market.St. Jude Medical:
Now part of Abbott Laboratories, St. Jude Medical has a strong presence in the cardiovascular market, providing advanced TPV solutions aimed at improving patient outcomes.Boston Scientific Corporation:
Another key player in the cardiovascular segment, Boston Scientific's innovative approaches to valve procedures are notable contributions to the TPV market landscape.We're grateful to work with incredible clients.









FAQs
What is the market size of transcatheter pulmonary valve?
The global transcatheter pulmonary valve market is projected to reach $1.5 billion by 2033, growing at a CAGR of 6.8%. This growth reflects increased adoption of minimally invasive procedures and advancements in valve technologies, meeting the rising demand for cardiac interventions.
What are the key market players or companies in this transcatheter pulmonary valve industry?
Key players in the transcatheter pulmonary valve market include Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott Laboratories. These companies lead in innovation, product development, and distribution, significantly contributing to market growth.
What are the primary factors driving the growth in the transcatheter pulmonary valve industry?
Significant growth factors include rising incidences of congenital heart diseases, technological advancements in valve design, and increasing preference for minimally invasive surgical procedures. Moreover, growing awareness and better healthcare infrastructure worldwide stimulate market expansion.
Which region is the fastest Growing in the transcatheter pulmonary valve?
North America is the fastest-growing region in the transcatheter pulmonary valve market, expected to grow from $0.54 billion in 2023 to $1.07 billion by 2033. Strong healthcare systems and advanced medical technologies contribute to this growth.
Does ConsaInsights provide customized market report data for the transcatheter pulmonary valve industry?
Yes, Consainsights offers customized market reports tailored to client needs in the transcatheter pulmonary valve industry. Clients can request specific data segments, geographical insights, and trend analyses for informed decision-making.
What deliverables can I expect from this transcatheter pulmonary valve market research project?
Clients can expect comprehensive reports including market size data, growth projections, competitive landscape analysis, segmentation insights, and regional performance metrics. These deliverables provide a thorough understanding of the market dynamics.
What are the market trends of transcatheter pulmonary valve?
Current trends include increasing innovations in valve technology, a shift towards home-based care models, and greater focus on patient-centric devices. The rise in regulatory approvals for novel products further supports market growth.