Transdermal Drug Delivery Systems Market Report
Published Date: 31 January 2026 | Report Code: transdermal-drug-delivery-systems
Transdermal Drug Delivery Systems Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Transdermal Drug Delivery Systems market for the forecast period of 2023 to 2033. It includes insights on market size, growth trends, segmentation, and competitive landscape, highlighting key innovations and regional dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
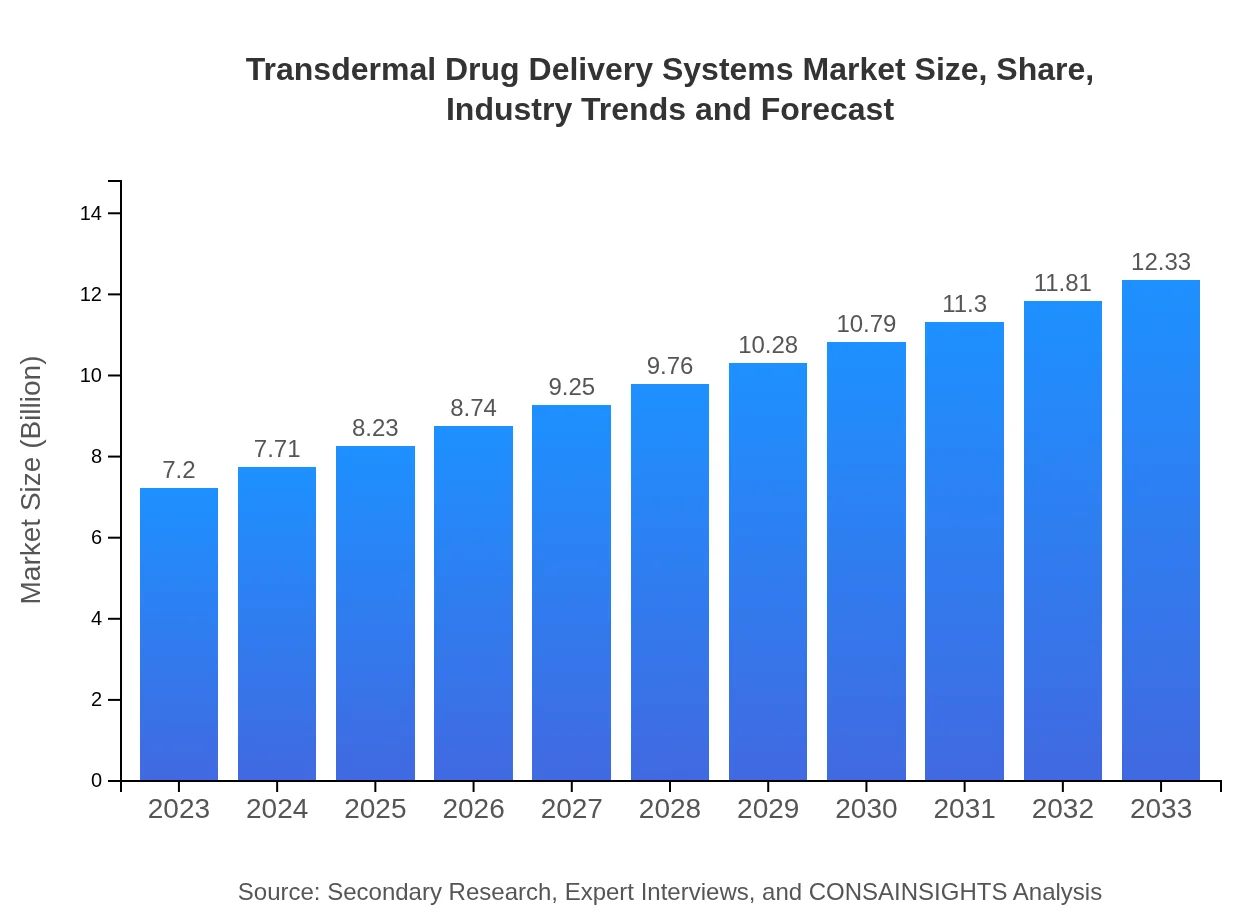
| 2023 Market Size | $7.20 Billion |
| CAGR (2023-2033) | 5.4% |
| 2033 Market Size | $12.33 Billion |
| Top Companies | Johnson & Johnson, Purdue Pharma, Mylan N.V., Teva Pharmaceutical Industries Ltd. |
| Last Modified Date | 31 January 2026 |
Transdermal Drug Delivery Systems Market Overview
Customize Transdermal Drug Delivery Systems Market Report market research report
- ✔ Get in-depth analysis of Transdermal Drug Delivery Systems market size, growth, and forecasts.
- ✔ Understand Transdermal Drug Delivery Systems's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Transdermal Drug Delivery Systems
What is the Market Size & CAGR of Transdermal Drug Delivery Systems market in 2023?
Transdermal Drug Delivery Systems Industry Analysis
Transdermal Drug Delivery Systems Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Transdermal Drug Delivery Systems Market Analysis Report by Region
Europe Transdermal Drug Delivery Systems Market Report:
Europe's market size is estimated to grow from 1.88 billion USD in 2023 to 3.21 billion USD by 2033. Factors such as an aging population, increased consumer awareness of the benefits of transdermal systems, and support from government bodies enhance market prospects.Asia Pacific Transdermal Drug Delivery Systems Market Report:
The Asia Pacific region is projected to grow significantly, with the market size estimated at 2.59 billion USD by 2033, up from 1.51 billion USD in 2023. This growth is attributed to the rising prevalence of chronic diseases and an increase in healthcare spending in emerging economies like China and India.North America Transdermal Drug Delivery Systems Market Report:
North America holds the largest share of the TDDS market, with a projected value of 4.58 billion USD by 2033, rising from 2.68 billion USD in 2023. The growth is primarily driven by advanced healthcare facilities, a high prevalence of chronic conditions, and a strong focus on research and development.South America Transdermal Drug Delivery Systems Market Report:
In South America, the market is expected to increase from 0.58 billion USD in 2023 to 0.99 billion USD by 2033. The demand for innovative drug delivery solutions is on the rise, facilitated by improved healthcare infrastructure and initiatives promoting advanced treatments.Middle East & Africa Transdermal Drug Delivery Systems Market Report:
The Middle East and Africa market is expected to expand significantly, with estimates increasing from 0.55 billion USD in 2023 to 0.95 billion USD by 2033. Growing investments in healthcare infrastructure and rising awareness about advanced drug delivery methods are key growth drivers.Tell us your focus area and get a customized research report.
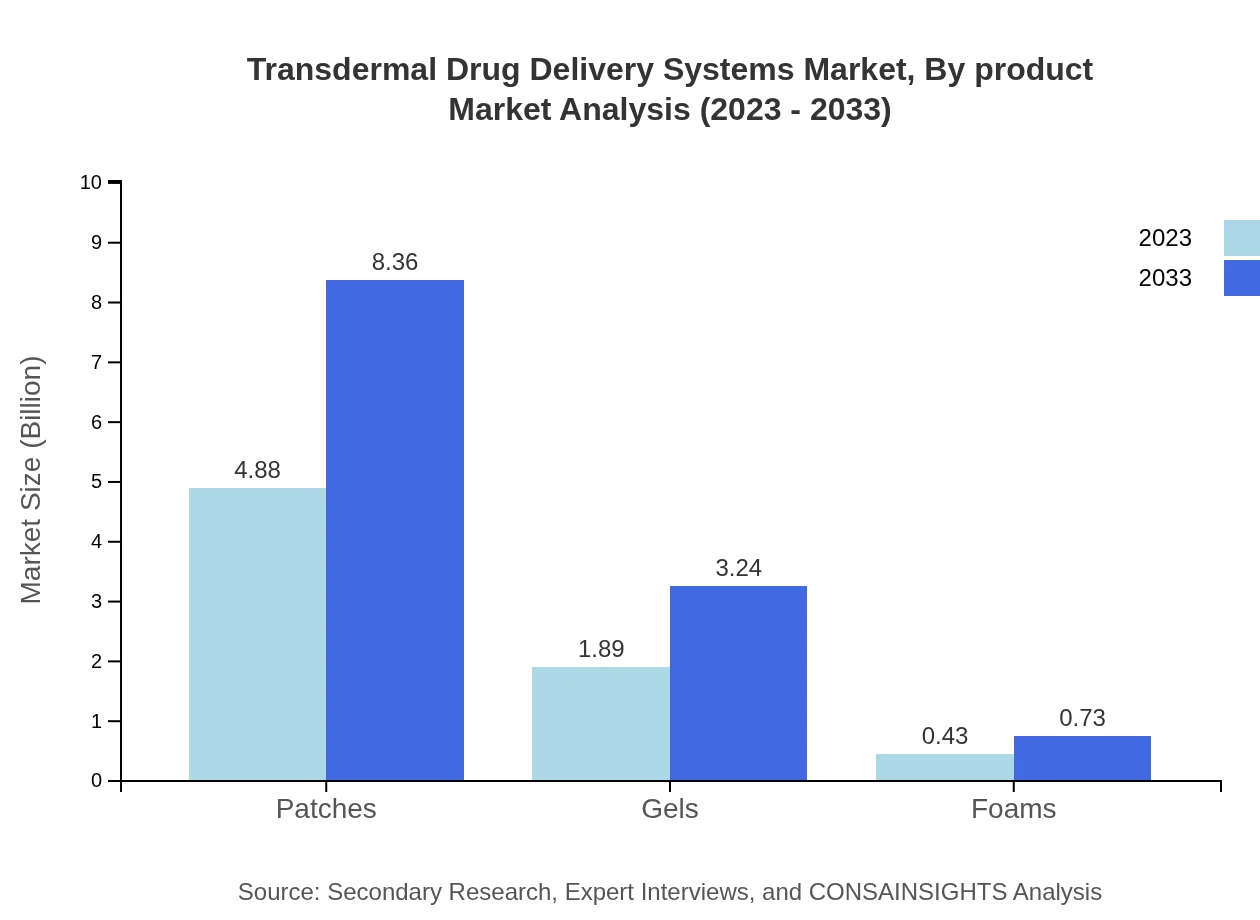
Transdermal Drug Delivery Systems Market Analysis By Product
The patch segment remains dominant, projected to reach 8.36 billion USD by 2033, maintaining a market share of 67.8%. It is followed by gels at 3.24 billion USD (26.25% share) and microneedle technology at 10.05 billion USD (81.55% share), showcasing varied applications across different demographics.
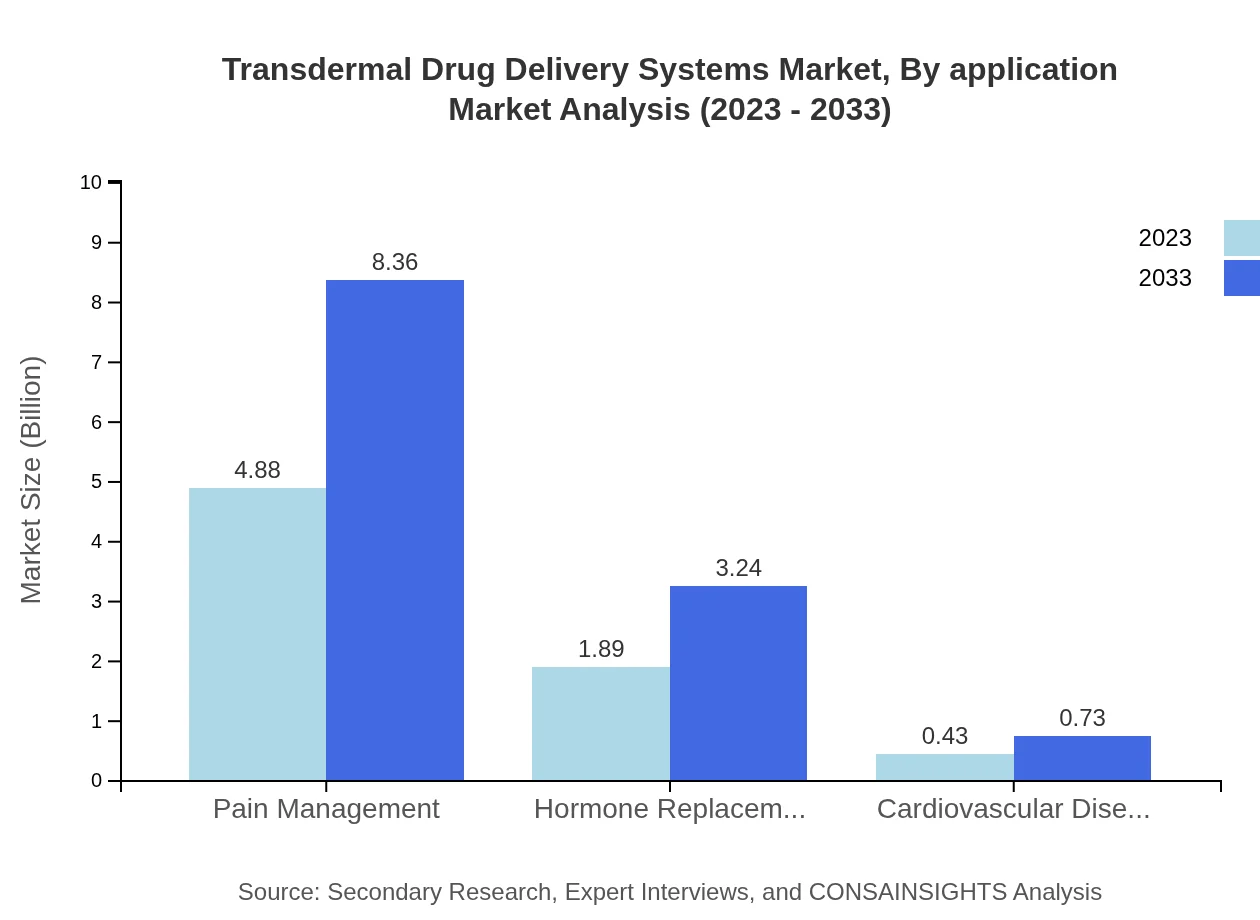
Transdermal Drug Delivery Systems Market Analysis By Application
The pain management segment is poised to grow significantly, achieving a size of 8.36 billion USD by 2033, maintaining a 67.8% market share, while hormone replacement therapy is expected to expand to 3.24 billion USD by 2033 (26.25% share).
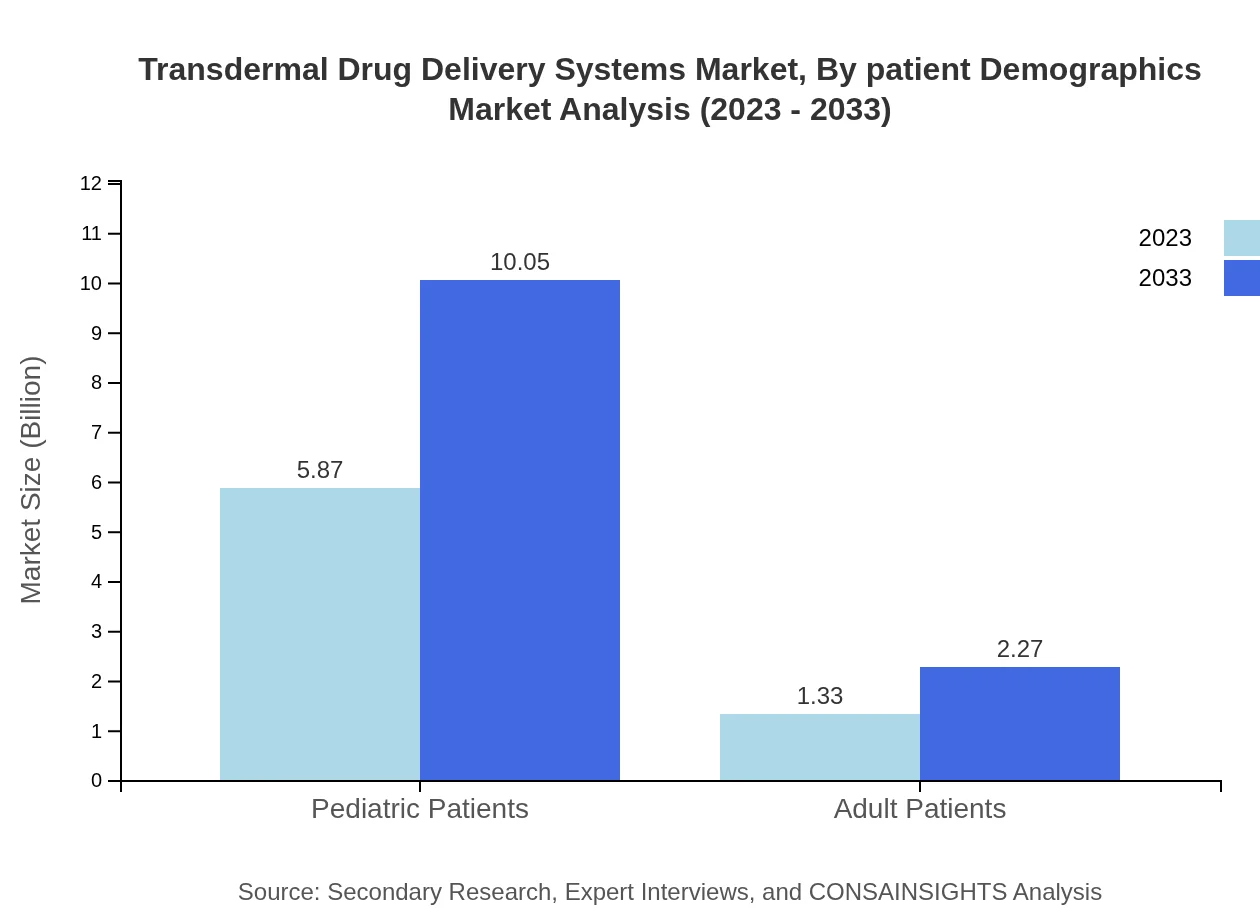
Transdermal Drug Delivery Systems Market Analysis By Patient Demographics
Pediatric patients are projected to dominate, with an estimated market size reaching 10.05 billion USD by 2033 (81.55%) due to the rising acceptance of non-invasive drug delivery systems, while adult patients will grow to 2.27 billion USD (18.45%).
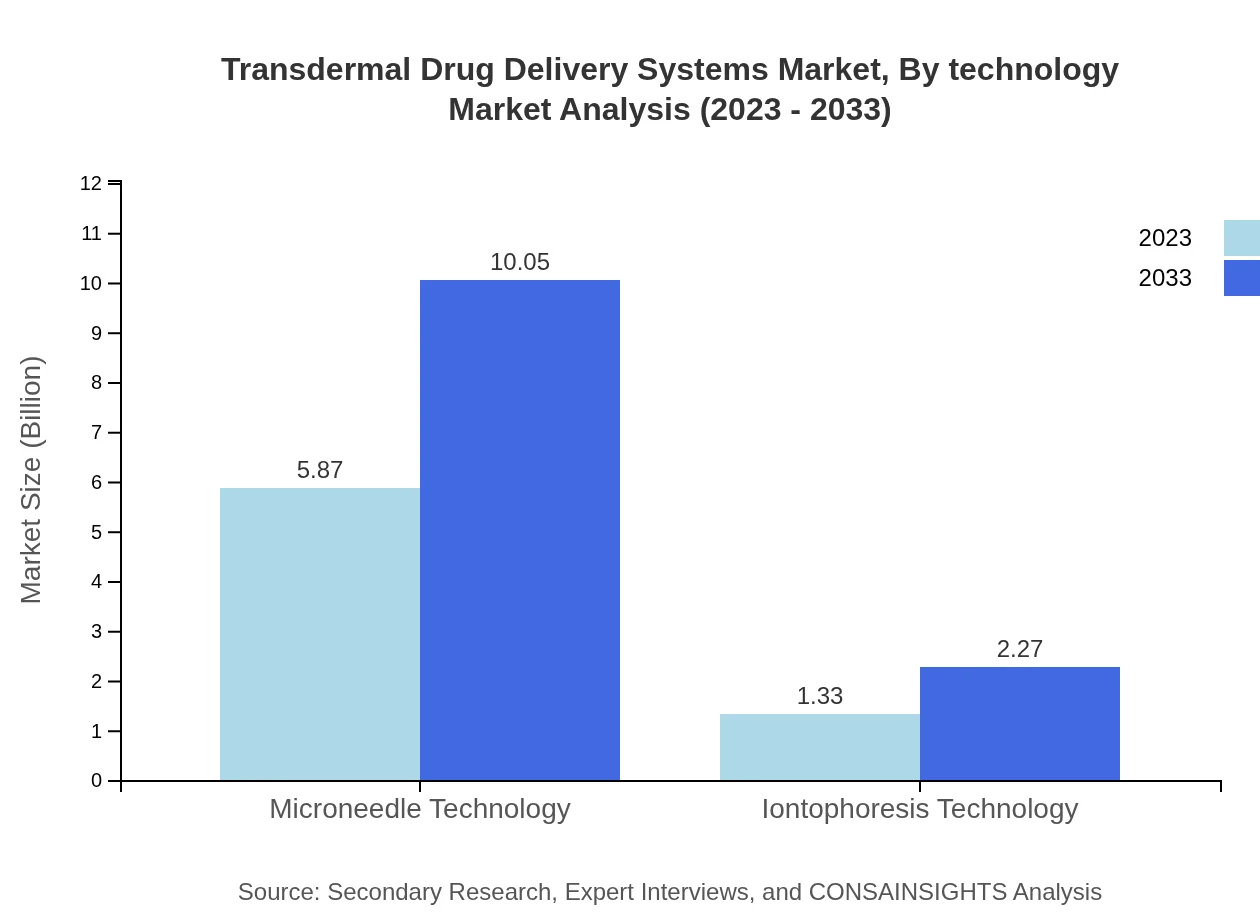
Transdermal Drug Delivery Systems Market Analysis By Technology
Innovative technologies such as microneedle and iontophoresis exhibit substantial growth potential, with microneedle technology expected to grow from 5.87 billion USD in 2023 to 10.05 billion USD by 2033 (81.55% share).
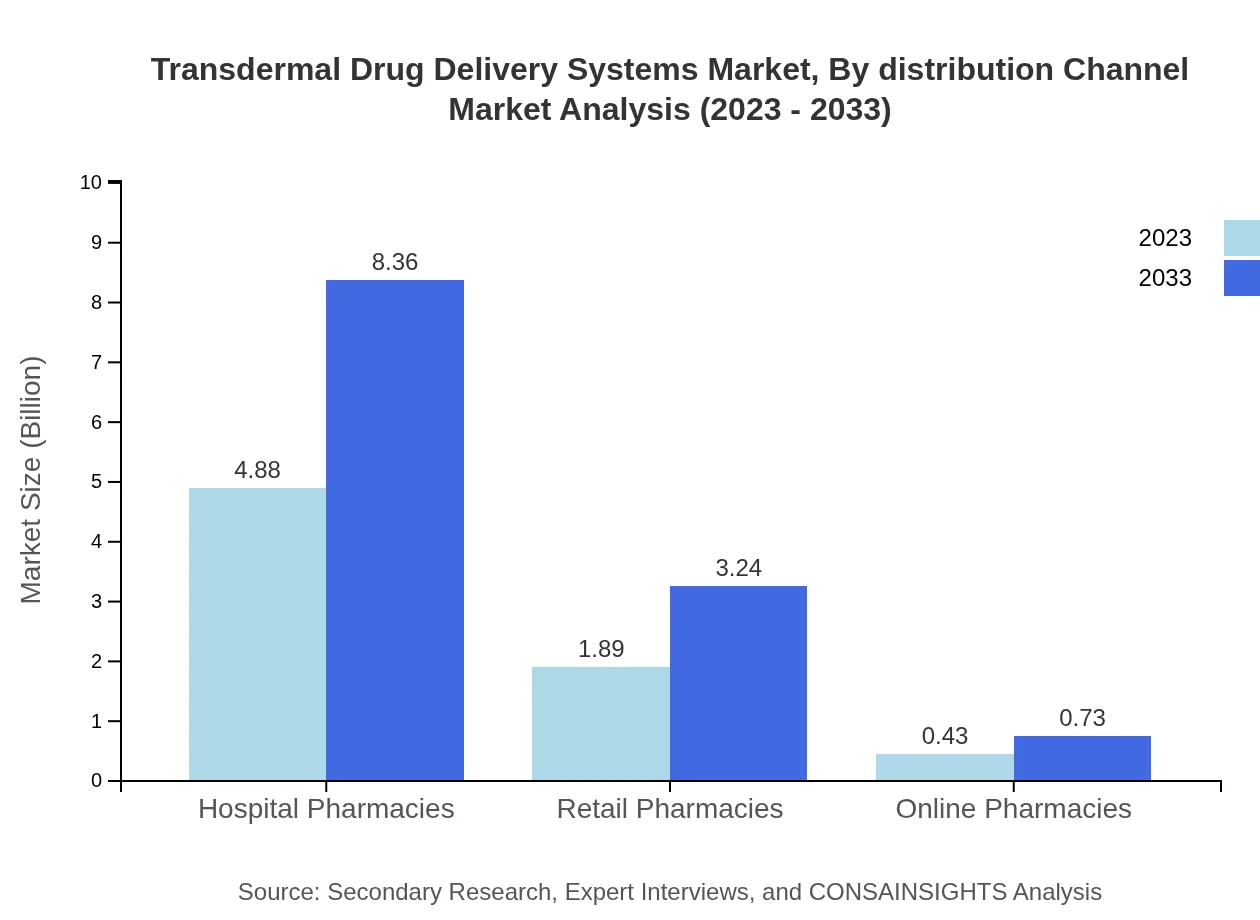
Transdermal Drug Delivery Systems Market Analysis By Distribution Channel
Hospital pharmacies remain the largest distribution channel, expected to attain 8.36 billion USD by 2033 (67.8% share), followed by retail and online pharmacies with markets sizes of 3.24 billion USD and 0.73 billion USD respectively.
Transdermal Drug Delivery Systems Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Transdermal Drug Delivery Systems Industry
Johnson & Johnson:
A leader in healthcare and pharmaceutical industries, Johnson & Johnson has developed innovative transdermal patches for pain management and hormone replacement therapies.Purdue Pharma:
Purdue Pharma specializes in the development of transdermal systems primarily focused on pain management solutions, leveraging advanced drug delivery technologies.Mylan N.V.:
Mylan offers a variety of transdermal products across several medical applications, ensuring patient compliance and effective drug delivery.Teva Pharmaceutical Industries Ltd.:
Teva is recognized for its extensive portfolio of generic and specialty medications, including transdermal delivery systems for chronic conditions.We're grateful to work with incredible clients.









FAQs
What is the market size of transdermal Drug Delivery Systems?
The transdermal drug delivery systems market is projected to grow from $7.2 billion in 2023 to an estimated size by 2033. The market is expected to achieve a CAGR of 5.4% during this period.
What are the key market players or companies in this transdermal Drug Delivery Systems industry?
Key players in the transdermal drug delivery systems market include Acorda Therapeutics, Inc., Hisamitsu Pharmaceutical Co., Inc., Johnson & Johnson, and Mylan N.V. These companies significantly contribute to innovation and market growth.
What are the primary factors driving the growth in the transdermal Drug Delivery Systems industry?
Factors driving the growth include increasing demand for minimally invasive drug delivery methods, advancements in technology, the rising prevalence of chronic diseases, and favorable regulatory frameworks supporting innovation.
Which region is the fastest Growing in the transdermal Drug Delivery Systems?
North America is currently the fastest-growing region in the transdermal drug delivery systems market, with a projected increase from $2.68 billion in 2023 to $4.58 billion by 2033.
Does Consainsights provide customized market report data for the transdermal Drug Delivery Systems industry?
Yes, Consainsights offers customized market report data tailored to specific needs in the transdermal drug delivery systems industry, ensuring relevant insights and analyses for stakeholders.
What deliverables can I expect from this transdermal Drug Delivery Systems market research project?
Deliverables from this market research project include comprehensive market reports, insights into trends and forecasts, competitor analysis, and segmented data across various categories including regions and product types.
What are the market trends of transdermal Drug Delivery Systems?
Current trends include the increasing adoption of microneedle technology, expanding applications in pain management and hormone replacement therapy, and a significant focus on pediatric patients utilizing transdermal systems.