Trauma Fixation Devices Market Report
Published Date: 31 January 2026 | Report Code: trauma-fixation-devices
Trauma Fixation Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Trauma Fixation Devices market, examining market size, growth potential, segmentation, regional insights, and emerging trends from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
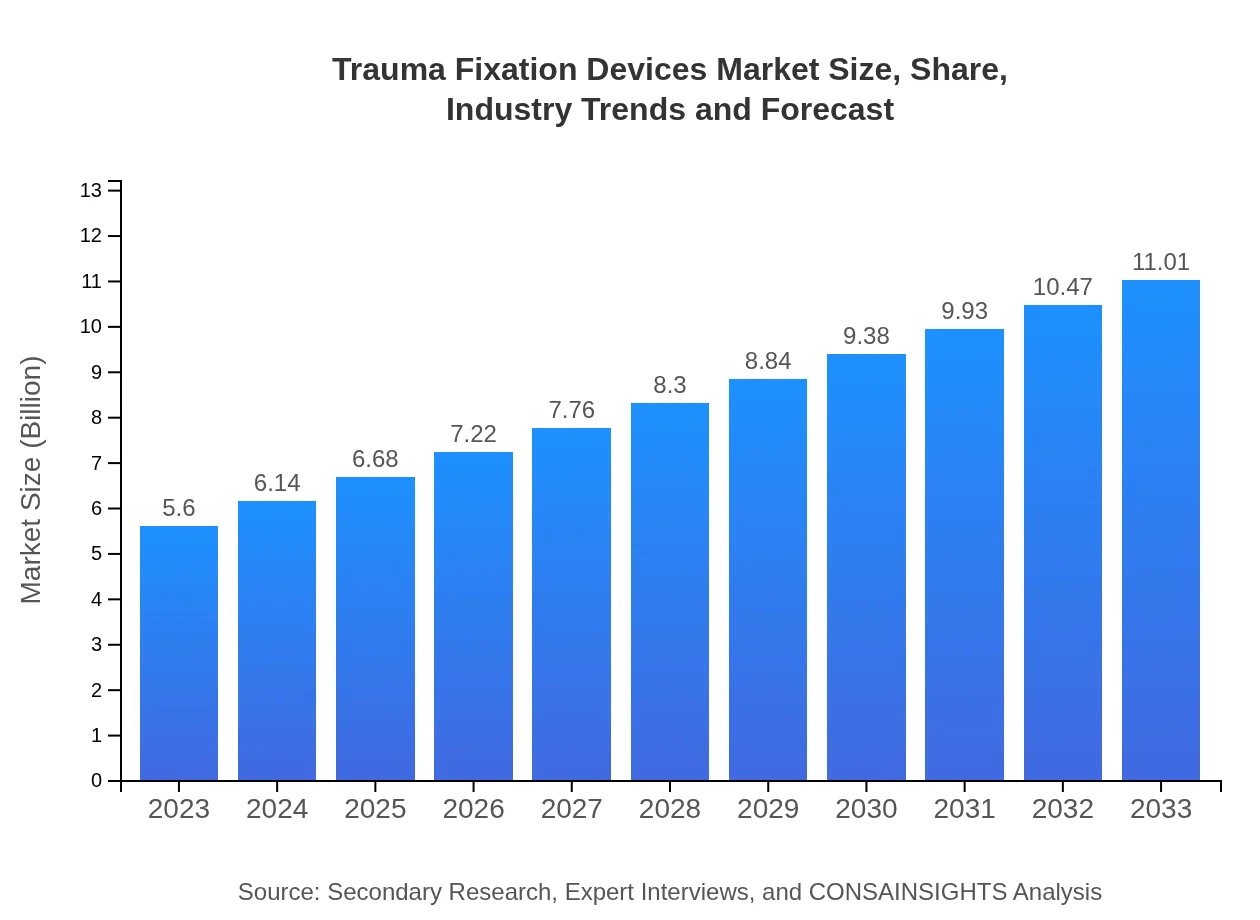
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $11.01 Billion |
| Top Companies | Medtronic , Stryker Corporation, Johnson & Johnson, Smith & Nephew |
| Last Modified Date | 31 January 2026 |
Trauma Fixation Devices Market Overview
Customize Trauma Fixation Devices Market Report market research report
- ✔ Get in-depth analysis of Trauma Fixation Devices market size, growth, and forecasts.
- ✔ Understand Trauma Fixation Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Trauma Fixation Devices
What is the Market Size & CAGR of Trauma Fixation Devices market in 2023?
Trauma Fixation Devices Industry Analysis
Trauma Fixation Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Trauma Fixation Devices Market Analysis Report by Region
Europe Trauma Fixation Devices Market Report:
Europe’s market for Trauma Fixation Devices stood at $1.58 billion in 2023 with a projected increase to $3.11 billion by 2033. Factors contributing to this include rising surgical procedures, advancements in fixation device designs, and higher health consciousness among the population.Asia Pacific Trauma Fixation Devices Market Report:
In the Asia Pacific region, the market for Trauma Fixation Devices was valued at $1.17 billion in 2023 and is expected to reach $2.30 billion by 2033, indicating significant growth driven by increasing accident rates and advancements in medical technology.North America Trauma Fixation Devices Market Report:
North America holds a substantial market share with an estimated value of $1.89 billion in 2023. The market is projected to grow to $3.72 billion by 2033, stimulated by high healthcare expenditure, advanced medical research, and a growing elderly population.South America Trauma Fixation Devices Market Report:
The South American market for Trauma Fixation Devices was valued at $0.34 billion in 2023, with an anticipated growth to $0.68 billion by 2033. Growth is supported by an increased focus on healthcare infrastructure and rising awareness of trauma care.Middle East & Africa Trauma Fixation Devices Market Report:
In the Middle East and Africa, the market size for Trauma Fixation Devices was around $0.61 billion in 2023 and is expected to double to $1.20 billion by 2033. This growth is primarily due to improving healthcare systems and increased access to orthopedic medical services.Tell us your focus area and get a customized research report.
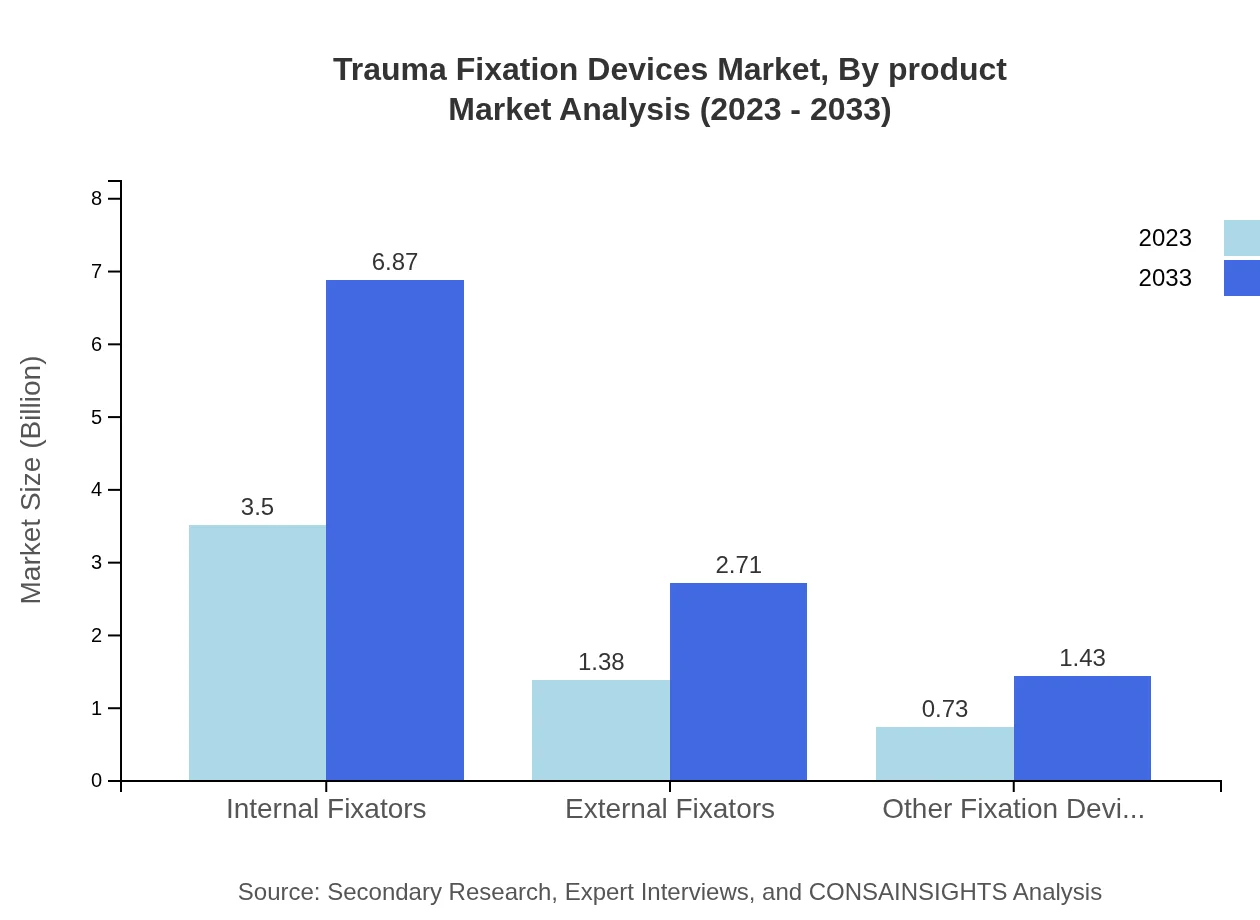
Trauma Fixation Devices Market Analysis By Product
In terms of product types, the Internal Fixators segment is the market leader with a size of $3.50 billion in 2023, expected to grow to $6.87 billion by 2033, holding a market share of 62.44%. External Fixators follow with a current value of $1.38 billion, projected to increase to $2.71 billion over the same period, representing 24.61% market share. Other fixation devices constitute the remaining share, reflecting their role in niche applications.
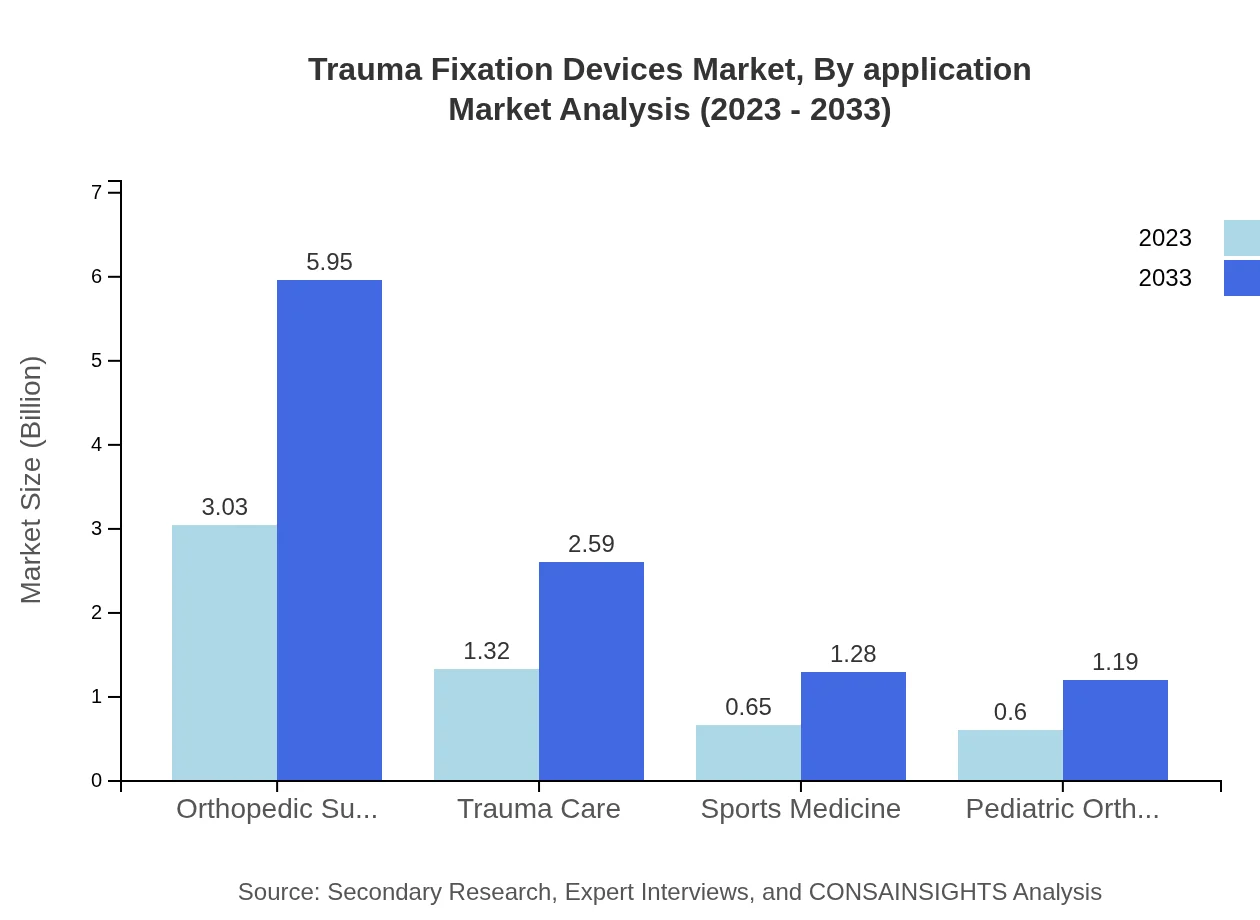
Trauma Fixation Devices Market Analysis By Application
The primary applications of Trauma Fixation Devices include Orthopedic Surgeries, Trauma Care, Sports Medicine, and Pediatric Orthopedics. Orthopedic Surgeries utilize the majority share at 54.02%, with a market size of $3.03 billion expected to reach $5.95 billion by 2033. Trauma Care devices represent 23.51% of the market, growing from $1.32 billion to $2.59 billion, while Sports Medicine and Pediatric Orthopedics account for smaller shares at 11.67% and 10.8%, respectively.
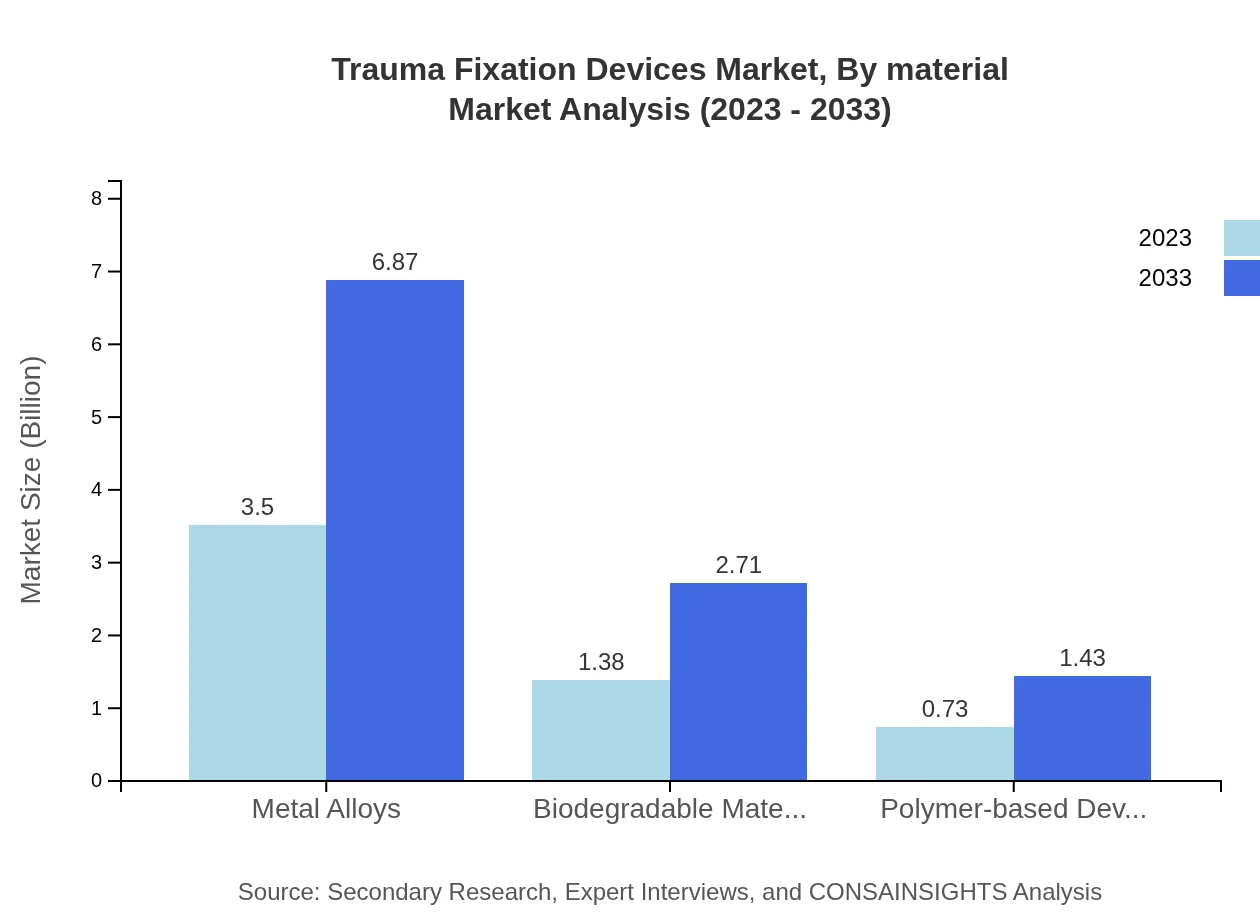
Trauma Fixation Devices Market Analysis By Material
The Trauma Fixation Devices market by material is segmented into Metal Alloys, Biodegradable Materials, and Polymer-based Devices. Metal Alloys dominate the market with a size of $3.50 billion (62.44% share). Biodegradable Materials and Polymer-based Devices follow with respective market sizes of $1.38 billion and $0.73 billion, seen as innovative alternatives driving growth due to their applications in specific surgical scenarios and patient preferences.
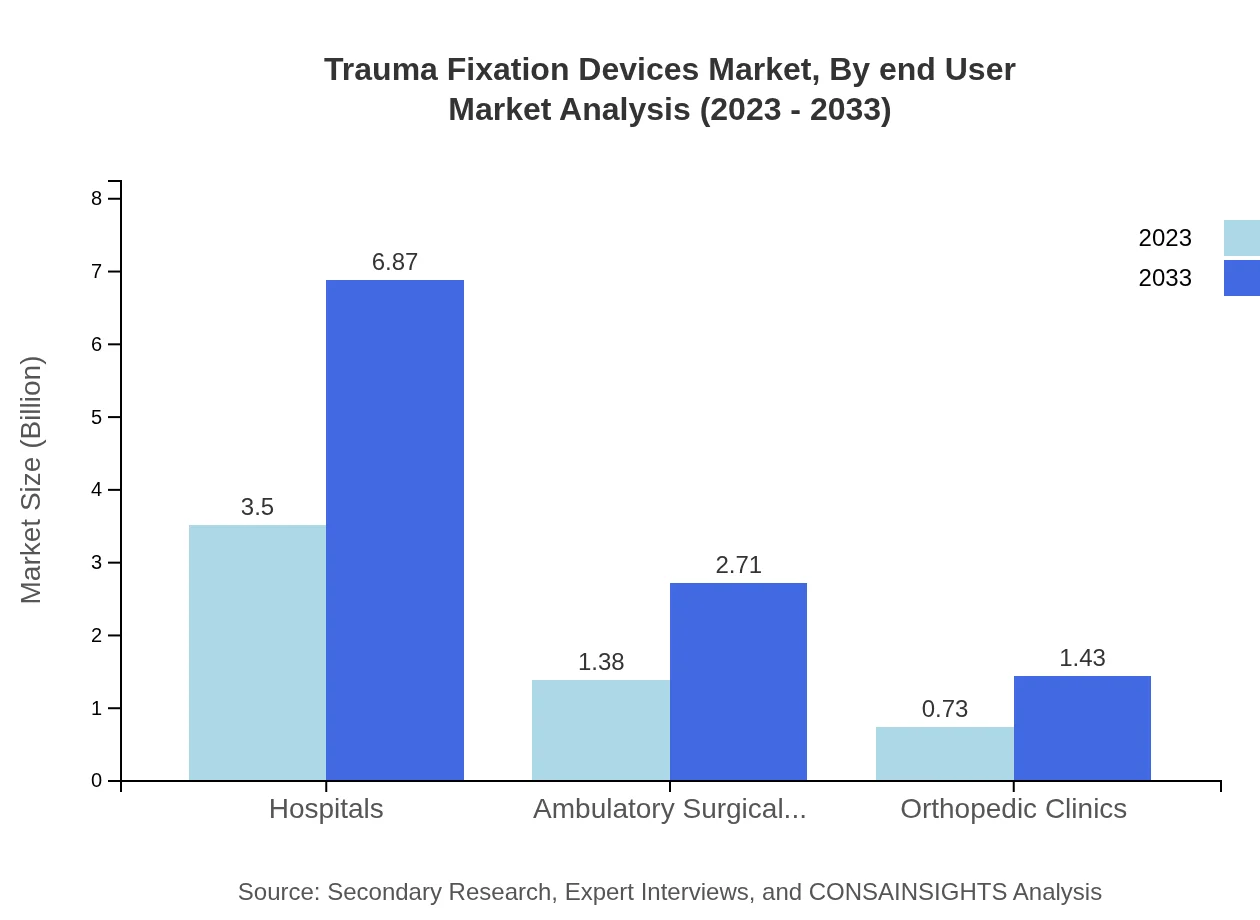
Trauma Fixation Devices Market Analysis By End User
The end-users include Hospitals, Ambulatory Surgical Centers, and Orthopedic Clinics. Hospitals hold the majority market share with 62.44%, equating to $3.50 billion in 2023, projected to reach $6.87 billion by 2033. Ambulatory Surgical Centers contribute significantly with 24.61% of the share, while Orthopedic Clinics cover the remaining market by addressing specific orthopedic needs.
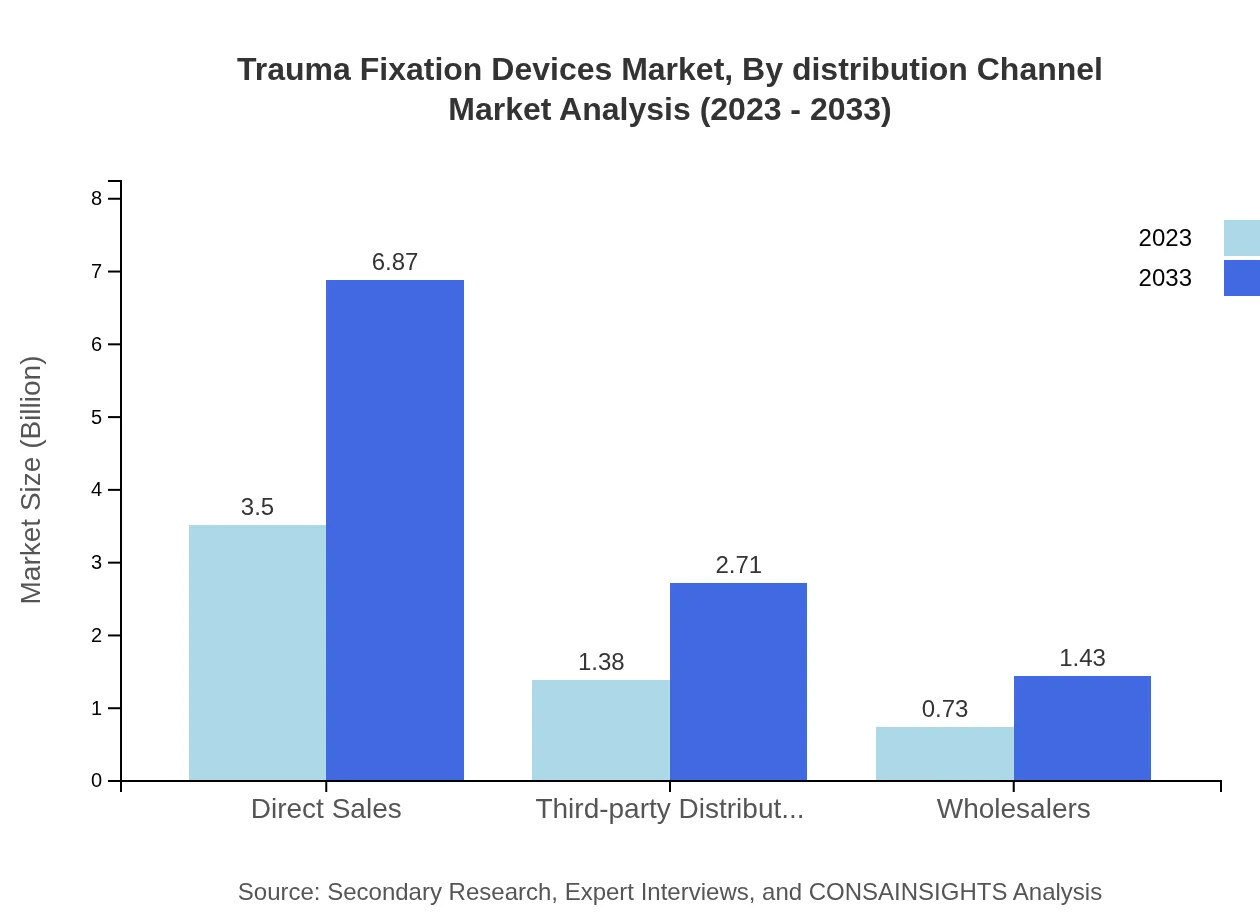
Trauma Fixation Devices Market Analysis By Distribution Channel
Direct Sales channels account for the largest share of the distribution, valued at $3.50 billion (62.44% share). Third-party Distributors represent an important segment with a 24.61% share, valued at $1.38 billion. Wholesalers cover the last segment, further enabling access to the Trauma Fixation Devices through various channels tailored to specific regions.
Trauma Fixation Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Trauma Fixation Devices Industry
Medtronic :
Medtronic is a global leader in medical technology, providing innovative Trauma Fixation Devices that enhance surgical outcomes and minimize recovery times.Stryker Corporation:
Stryker Corporation specializes in orthopedic products and has a significant range of trauma fixation solutions that focus on advanced technology and patient safety.Johnson & Johnson:
Johnson & Johnson is renowned for its medical devices and services, including advanced fixation devices that cater to various trauma care needs globally.Smith & Nephew:
Smith & Nephew offers a broad range of trauma fixation devices designed to promote healing and restore mobility for patients recovering from fractures.We're grateful to work with incredible clients.









FAQs
What is the market size of trauma Fixation Devices?
The trauma fixation devices market is projected to reach approximately $5.6 billion by 2033, growing at a CAGR of 6.8% from 2023. This growth is driven by increased incidents of traumatic injuries globally and advancements in medical technologies.
What are the key market players or companies in this trauma Fixation Devices industry?
Key players in the trauma fixation devices market include Medtronic, Johnson & Johnson, Stryker Corporation, Zimmer Biomet, and DePuy Synthes. These companies focus on innovation, strategic collaborations, and expanding their product portfolios to capture more market share.
What are the primary factors driving the growth in the trauma Fixation Devices industry?
The growth of the trauma fixation devices industry is fueled by factors such as the rising incidence of road accidents, increasing sports injuries, an aging population susceptible to fractures, and advancements in minimally invasive surgical techniques.
Which region is the fastest Growing in the trauma Fixation Devices?
The Asia-Pacific region is the fastest-growing market for trauma fixation devices, with market size expected to increase from $1.17 billion in 2023 to $2.30 billion in 2033. Rapid urbanization and improved healthcare infrastructure contribute significantly to this growth.
Does ConsaInsights provide customized market report data for the trauma Fixation Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the trauma fixation devices industry. Clients can request analysis based on unique parameters such as region, segment, and competitive landscape.
What deliverables can I expect from this trauma Fixation Devices market research project?
Deliverables from this project include comprehensive market analysis reports, segment-wise breakdowns, regional insights, competitive landscape evaluations, and forecasting data for market growth, enabling informed decision-making.
What are the market trends of trauma Fixation Devices?
Current trends in the trauma fixation devices market include a shift towards biodegradable materials, growing adoption of advanced fixation technologies, increased investments in R&D, and a focus on patient-centered care in trauma management.