Tuberculosis Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: tuberculosis-diagnostics
Tuberculosis Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Tuberculosis Diagnostics market, providing in-depth insights and data forecasts for the period 2023 - 2033. It offers a comprehensive analysis of current market trends, regional performance, segmentation, and competitive landscape to aid stakeholders in strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
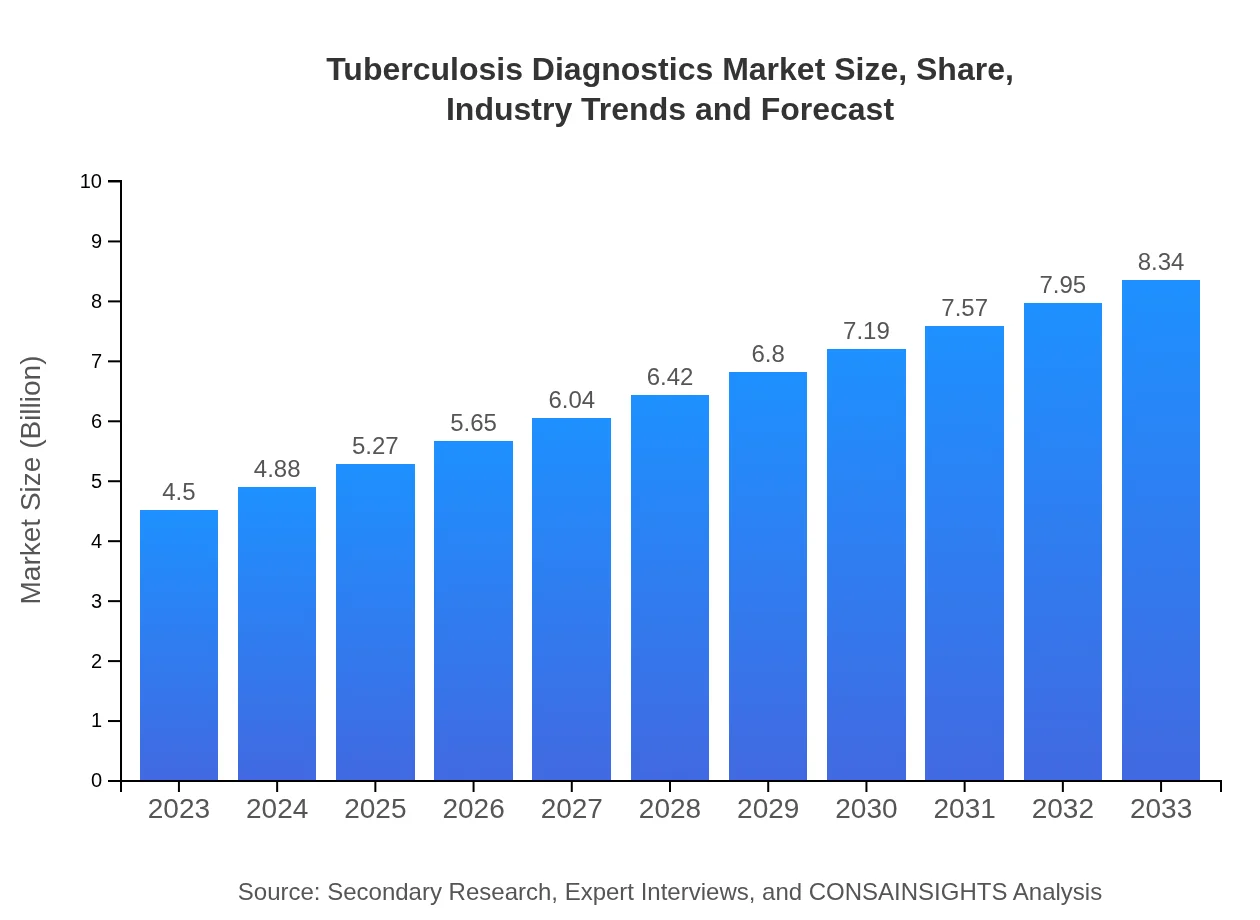
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $8.34 Billion |
| Top Companies | Roche Diagnostics, Thermo Fisher Scientific, Becton, Dickinson and Company (BD), Cepheid |
| Last Modified Date | 31 January 2026 |
Tuberculosis Diagnostics Market Overview
Customize Tuberculosis Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Tuberculosis Diagnostics market size, growth, and forecasts.
- ✔ Understand Tuberculosis Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Tuberculosis Diagnostics
What is the Market Size & CAGR of Tuberculosis Diagnostics market in 2023?
Tuberculosis Diagnostics Industry Analysis
Tuberculosis Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Tuberculosis Diagnostics Market Analysis Report by Region
Europe Tuberculosis Diagnostics Market Report:
Europe’s Tuberculosis Diagnostics market is anticipated to grow from $1.53 billion in 2023 to $2.83 billion by 2033, supported by favorable regulatory environments and investment in health technologies to combat TB effectively.Asia Pacific Tuberculosis Diagnostics Market Report:
In 2023, the Tuberculosis Diagnostics market in the Asia Pacific region is valued at approximately $0.82 billion, with projections reaching around $1.51 billion by 2033. The region’s growth is driven by high TB prevalence and significant investments in healthcare infrastructure, alongside rising demand for advanced diagnostic tools.North America Tuberculosis Diagnostics Market Report:
North America exhibits a strong market presence, pegged at $1.53 billion in 2023 and expected to expand to $2.83 billion by 2033. The rise is attributed to advanced healthcare systems, a strong focus on research and development, and a growing awareness of TB screenings.South America Tuberculosis Diagnostics Market Report:
The South American market for Tuberculosis Diagnostics is valued at about $0.28 billion in 2023 and is expected to rise to $0.52 billion by 2033. Key factors include increasing government initiatives for TB control and improvement in healthcare services within the region.Middle East & Africa Tuberculosis Diagnostics Market Report:
The Tuberculosis Diagnostics market in the Middle East and Africa holds a value of approximately $0.35 billion in 2023, with expectations to reach $0.65 billion by 2033. Rising healthcare challenges due to prevalent TB cases necessitate enhanced diagnostic capabilities across the region.Tell us your focus area and get a customized research report.
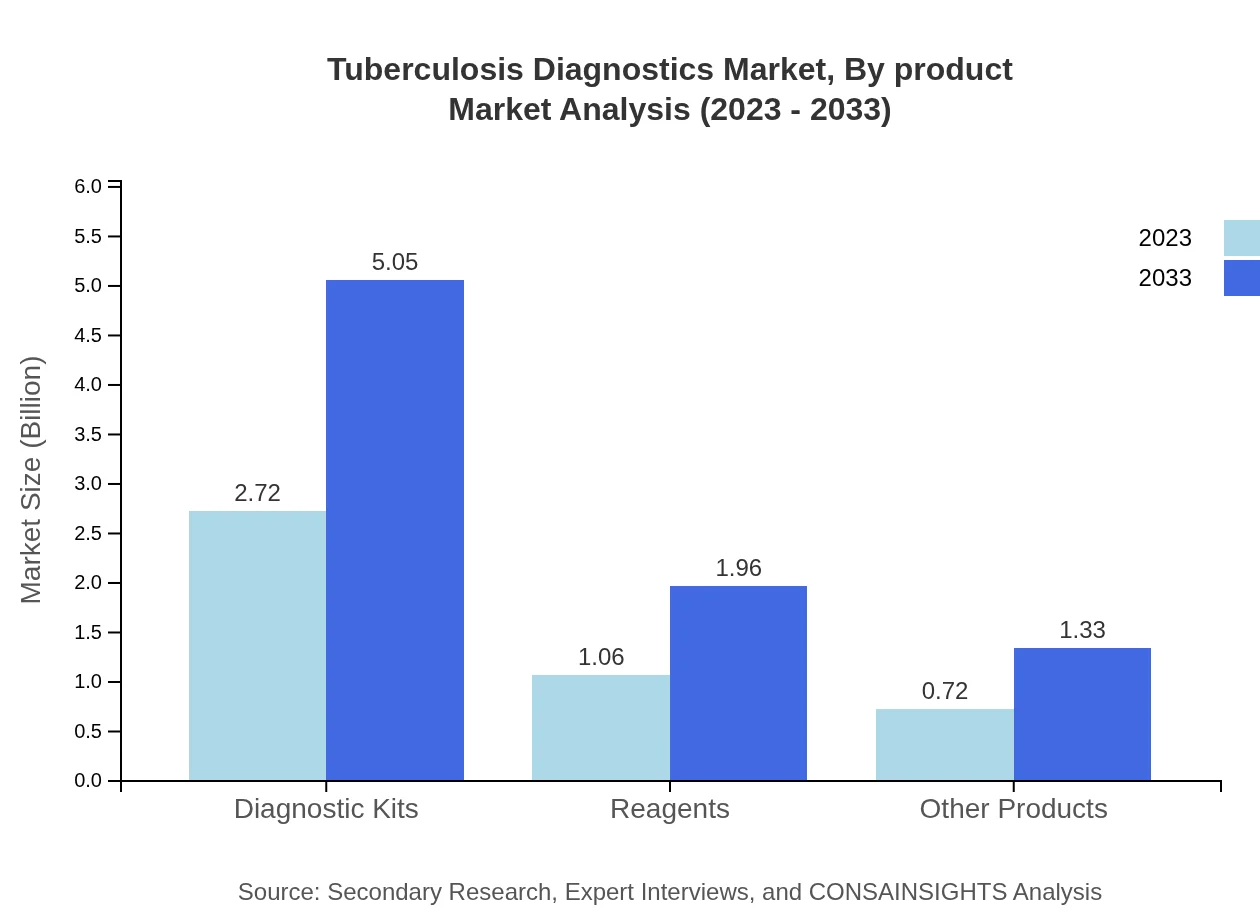
Tuberculosis Diagnostics Market Analysis By Product
In 2023, the market for Diagnostic Kits stands at $2.72 billion, representing 60.53% share, and is projected to reach $5.05 billion by 2033. Similarly, reagents account for $1.06 billion (23.47% share), growing to $1.96 billion. Other products, including consumables, held $0.72 billion (16% share) in 2023, expected to rise to $1.33 billion.
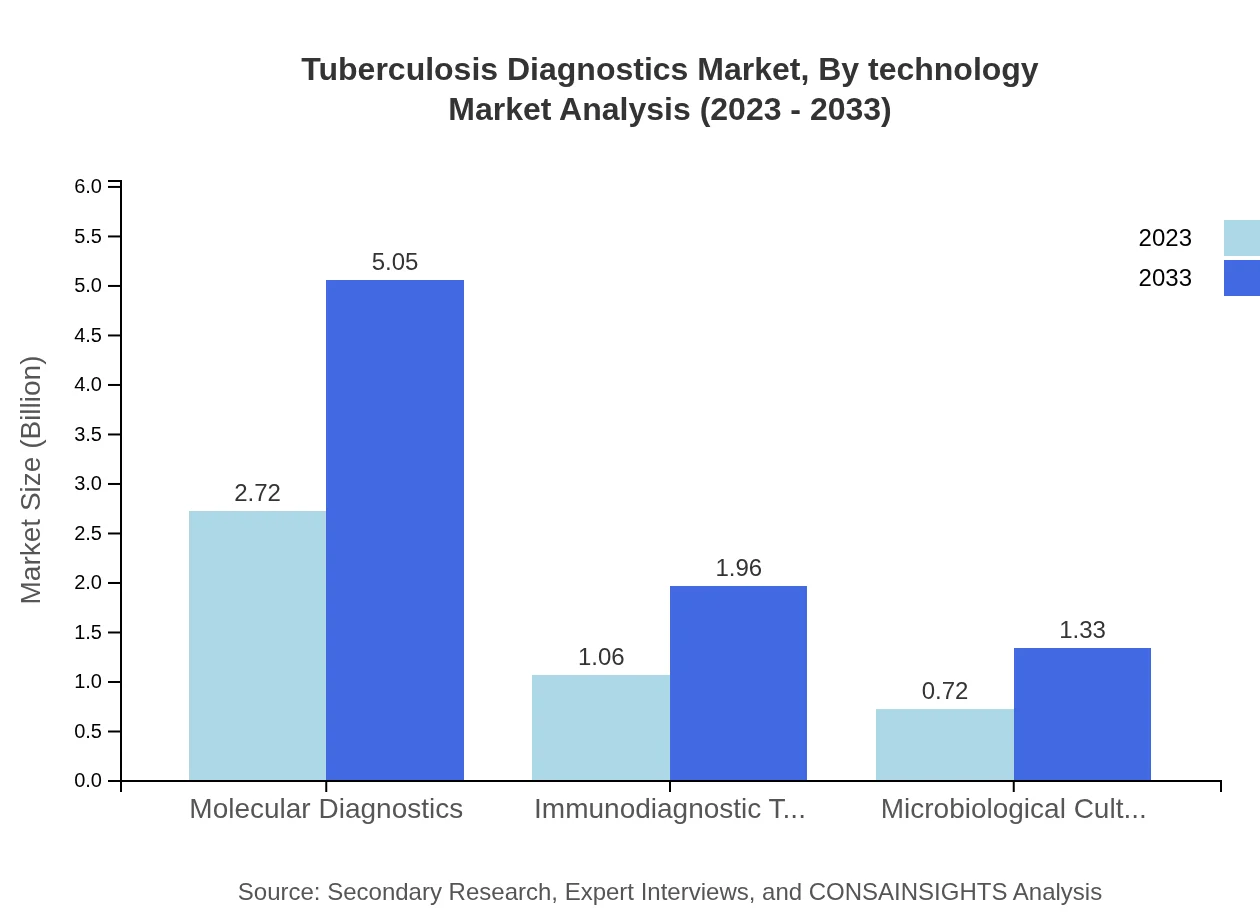
Tuberculosis Diagnostics Market Analysis By Technology
Molecular Diagnostics continues to lead the Tuberculosis Diagnostics market with a size of $2.72 billion (60.53% share) in 2023, anticipated to grow to $5.05 billion by 2033. Immunodiagnostic tests and microbiological cultures follow with $1.06 billion (23.47%) and $0.72 billion (16%) respectively in 2023.
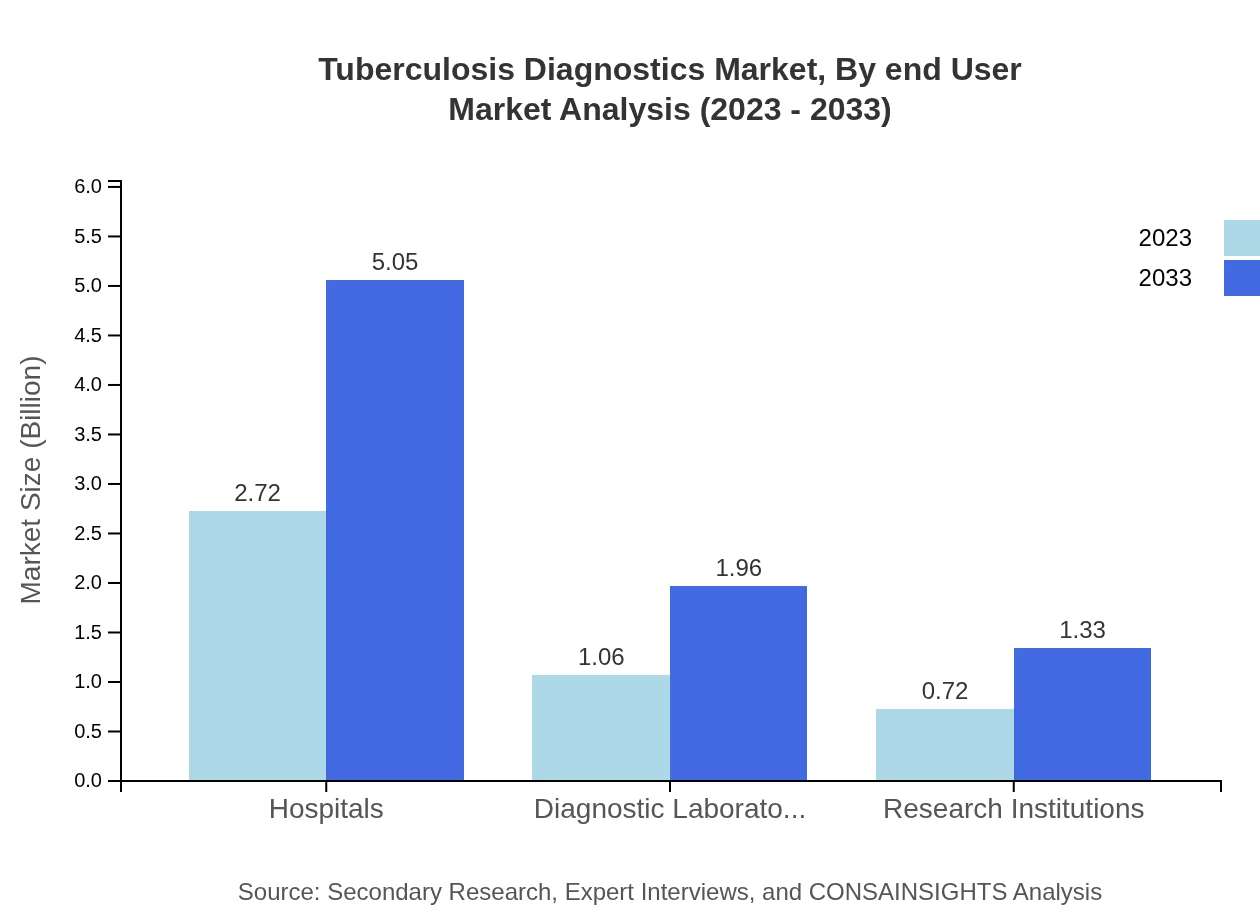
Tuberculosis Diagnostics Market Analysis By End User
Hospitals account for a significant portion of the Tuberculosis Diagnostics market, valued at $2.72 billion (60.53% share), projected to reach $5.05 billion by 2033. Diagnostic laboratories and research institutions cover $1.06 billion (23.47%) and $0.72 billion (16%) respectively in 2023.
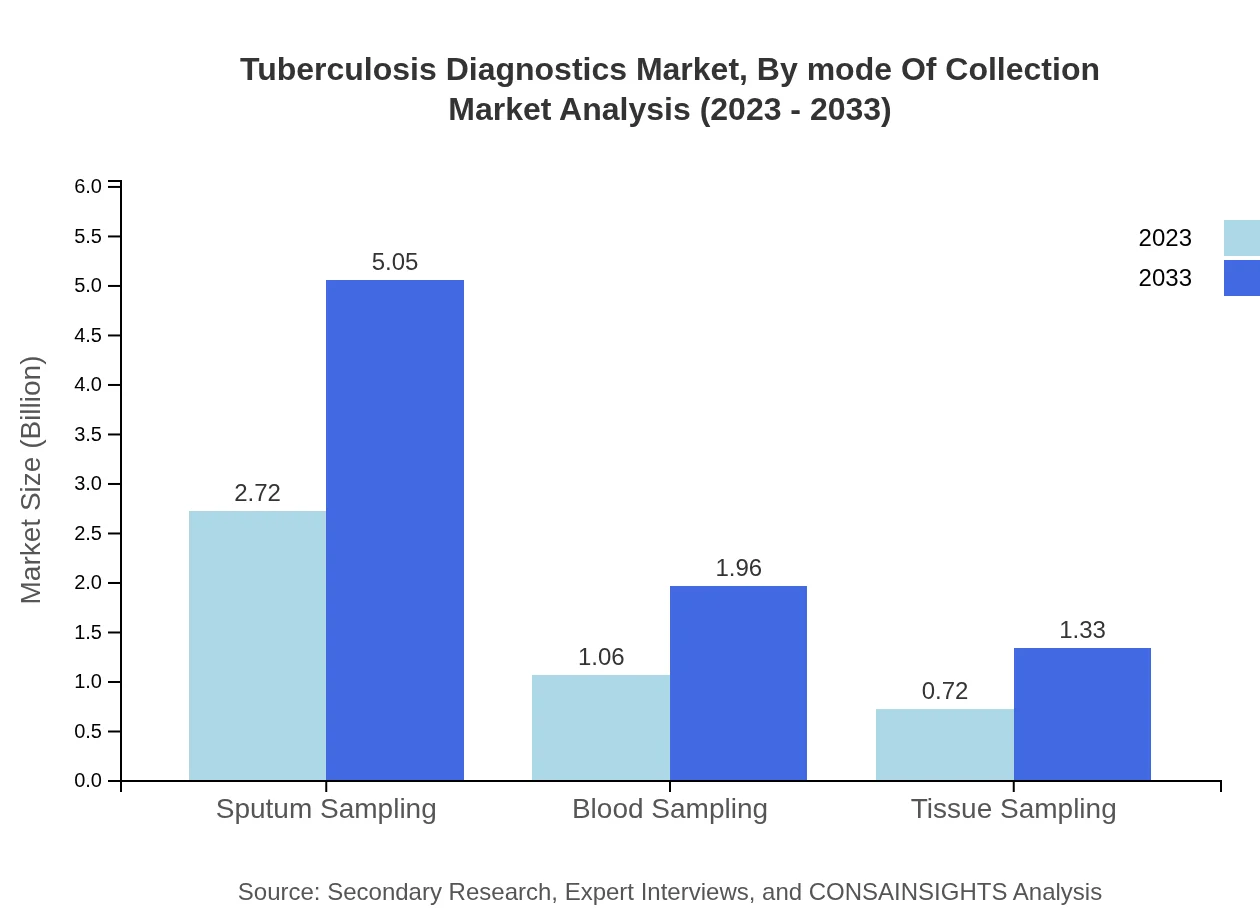
Tuberculosis Diagnostics Market Analysis By Mode Of Collection
Sputum sampling dominates the market at $2.72 billion (60.53% share) in 2023, with projections growing to $5.05 billion by 2033. Blood sampling and tissue sampling follow, holding $1.06 billion (23.47%) and $0.72 billion (16%) respectively.
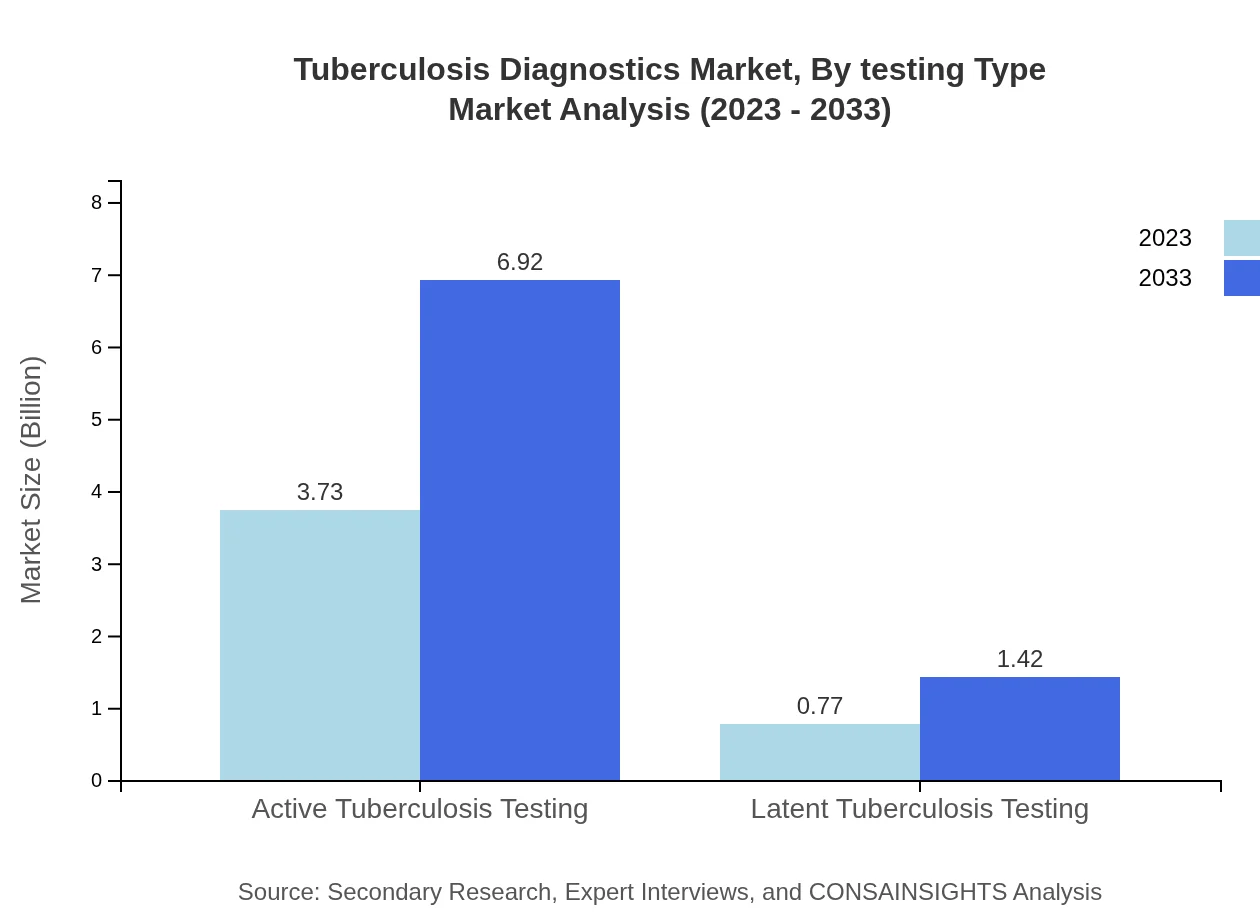
Tuberculosis Diagnostics Market Analysis By Testing Type
Active Tuberculosis Testing reaps the largest market share with $3.73 billion (82.94% share) in 2023, expected to rise to $6.92 billion by 2033. Latent Tuberculosis Testing is valued at $0.77 billion (17.06%) in 2023 and is projected to reach $1.42 billion.
Tuberculosis Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Tuberculosis Diagnostics Industry
Roche Diagnostics:
A leading company in innovative diagnostic solutions, Roche has been pivotal in developing advanced TB diagnostic tools, enhancing the timeliness and effectiveness of testing.Thermo Fisher Scientific:
Renowned for its extensive range of diagnostic products, Thermo Fisher focuses on delivering high-quality reagents and diagnostic kits crucial for TB testing.Becton, Dickinson and Company (BD):
BD has a significant footprint in the diagnostics space, known for its cutting-edge technologies in TB diagnostics that improve patient outcomes.Cepheid:
Innovator in molecular diagnostics, Cepheid is recognized for its rapid testing devices for TB, which have transformed TB management strategies globally.We're grateful to work with incredible clients.









FAQs
What is the market size of tuberculosis Diagnostics?
The tuberculosis diagnostics market is valued at approximately $4.5 billion in 2023, with an expected compound annual growth rate (CAGR) of 6.2%, projected to grow significantly over the next decade.
What are the key market players or companies in this tuberculosis Diagnostics industry?
Key players in the tuberculosis diagnostics market include major medical technology companies, diagnostic kit manufacturers, and biotech firms that innovate in diagnostics, focusing on quick and efficient tuberculosis detection.
What are the primary factors driving the growth in the tuberculosis Diagnostics industry?
The growth of the tuberculosis diagnostics industry is driven by increasing tuberculosis prevalence, government initiatives for disease control, advancements in diagnostic technologies, and rising healthcare investments worldwide.
Which region is the fastest Growing in the tuberculosis Diagnostics?
The Asia Pacific region is expected to be the fastest-growing in the tuberculosis diagnostics market, with an increase from $0.82 billion in 2023 to $1.51 billion by 2033, reflecting a strong demand for diagnostics.
Does ConsaInsights provide customized market report data for the tuberculosis Diagnostics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs and insights for stakeholders in the tuberculosis diagnostics industry, enhancing decision-making processes.
What deliverables can I expect from this tuberculosis Diagnostics market research project?
Deliverables from the tuberculosis diagnostics market research project include comprehensive reports, market analyses, forecasting data, competitive landscapes, and actionable insights customized to client requirements.
What are the market trends of tuberculosis Diagnostics?
Trends in the tuberculosis diagnostics market include the rise of molecular diagnostics, increased preference for rapid testing solutions, and evolving technologies in sample collection methods, enhancing efficiency and accuracy.