Umbilical Vessel Catheters Market Report
Published Date: 31 January 2026 | Report Code: umbilical-vessel-catheters
Umbilical Vessel Catheters Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Umbilical Vessel Catheters market, focusing on its size, growth forecasts, segmentation, technology trends, and an overview of key regional markets from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
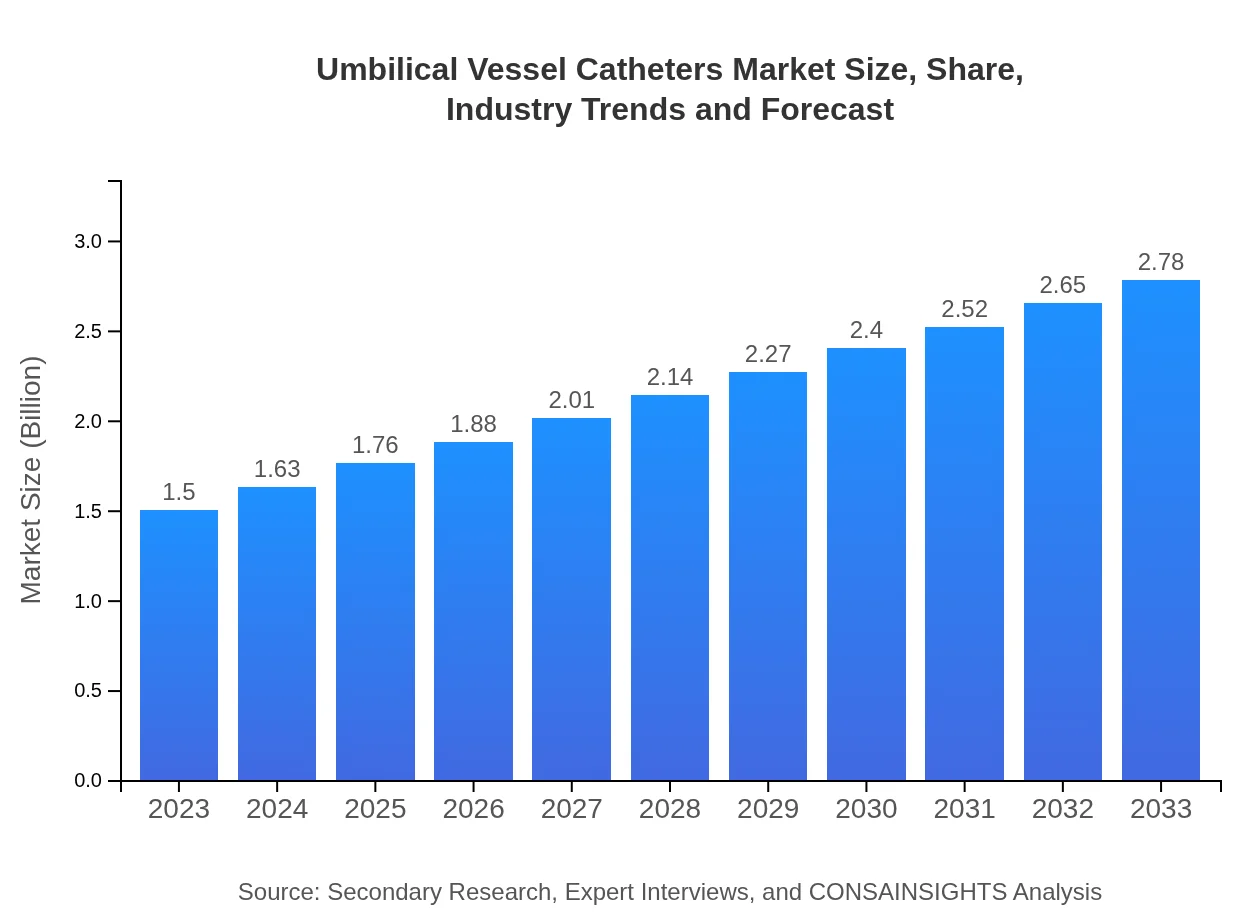
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $2.78 Billion |
| Top Companies | Medtronic , Becton, Dickinson and Company (BD), Vygon, Systagenix Wound Management |
| Last Modified Date | 31 January 2026 |
Umbilical Vessel Catheters Market Overview
Customize Umbilical Vessel Catheters Market Report market research report
- ✔ Get in-depth analysis of Umbilical Vessel Catheters market size, growth, and forecasts.
- ✔ Understand Umbilical Vessel Catheters's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Umbilical Vessel Catheters
What is the Market Size & CAGR of Umbilical Vessel Catheters market in 2023 and 2033?
Umbilical Vessel Catheters Industry Analysis
Umbilical Vessel Catheters Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Umbilical Vessel Catheters Market Analysis Report by Region
Europe Umbilical Vessel Catheters Market Report:
Similarly, Europe’s market is valued at $0.40 billion in 2023 and projected at $0.74 billion by 2033. The region benefits from highly developed healthcare systems and a rising number of neonatal cases requiring advanced care interventions.Asia Pacific Umbilical Vessel Catheters Market Report:
In Asia Pacific, the market is valued at $0.33 billion in 2023 and is expected to reach $0.61 billion by 2033, demonstrating a growth driven by increased healthcare access and rising birth rates. Countries like China and India are primary contributors owing to their large population base.North America Umbilical Vessel Catheters Market Report:
North America leads the market with a value of $0.48 billion in 2023 and is projected to expand to $0.89 billion by 2033. High healthcare expenditure, advanced neonatal care facilities, and a strong base of key manufacturers drive this growth.South America Umbilical Vessel Catheters Market Report:
The South American market is anticipated to grow from $0.14 billion in 2023 to $0.26 billion by 2033, fostered by improvements in healthcare infrastructure and growing neonatal care awareness across countries like Brazil and Argentina.Middle East & Africa Umbilical Vessel Catheters Market Report:
The Middle East and Africa market is expected to grow from $0.15 billion in 2023 to $0.28 billion by 2033, driven by enhancements in healthcare policies and the presence of international health organizations aiming for better newborn care.Tell us your focus area and get a customized research report.
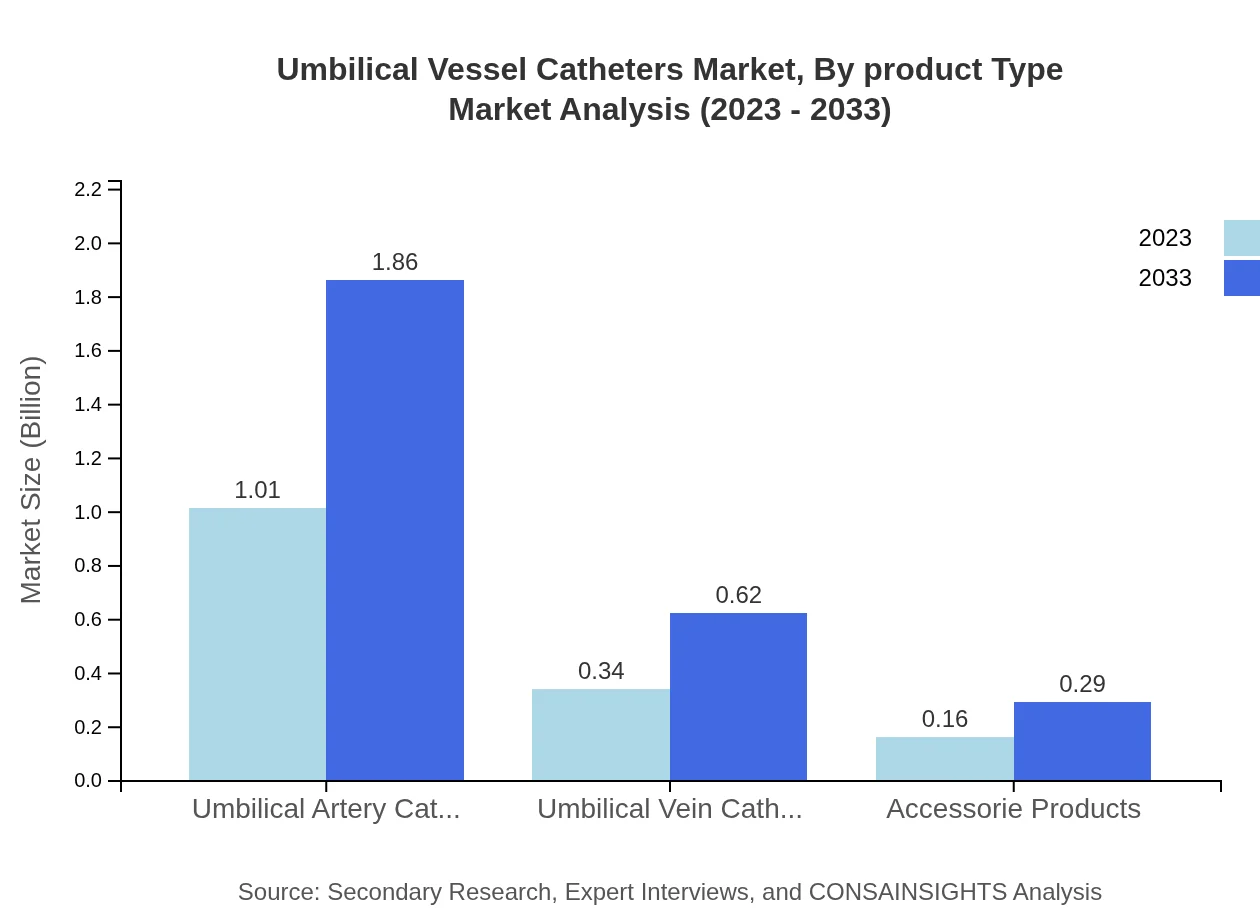
Umbilical Vessel Catheters Market Analysis By Product Type
The market for umbilical vessel catheters is significantly driven by two primary types: Umbilical Artery Catheters (UAC) and Umbilical Vein Catheters (UVC). The UAC segment has a market size of $1.01 billion in 2023, expected to grow to $1.86 billion by 2033, maintaining a 67.02% market share. Conversely, UVC has a market size of $0.34 billion in 2023, projected to grow to $0.62 billion by 2033, accounting for 22.38% of the market share.
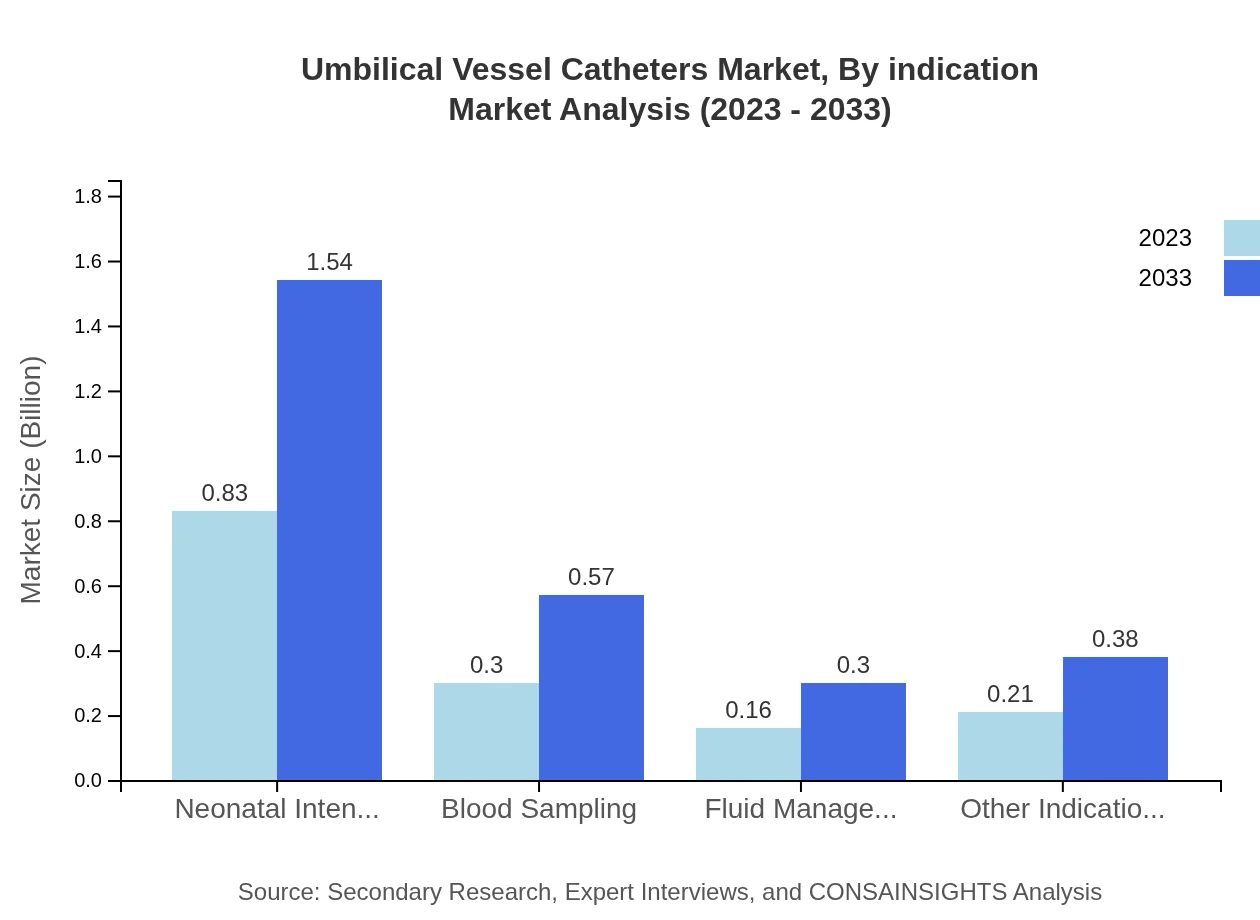
Umbilical Vessel Catheters Market Analysis By Indication
By indication, the Neonatal Intensive Care segment leads with a market size reflecting $0.83 billion in 2023 and expected to increase to $1.54 billion by 2033, holding a 55.35% market share. Other significant indications include Blood Sampling, which has a market size of $0.30 billion in 2023 (growing to $0.57 billion by 2033), and Fluid Management, which reflects a market size of $0.16 billion, expected to rise to $0.30 billion by the same year.
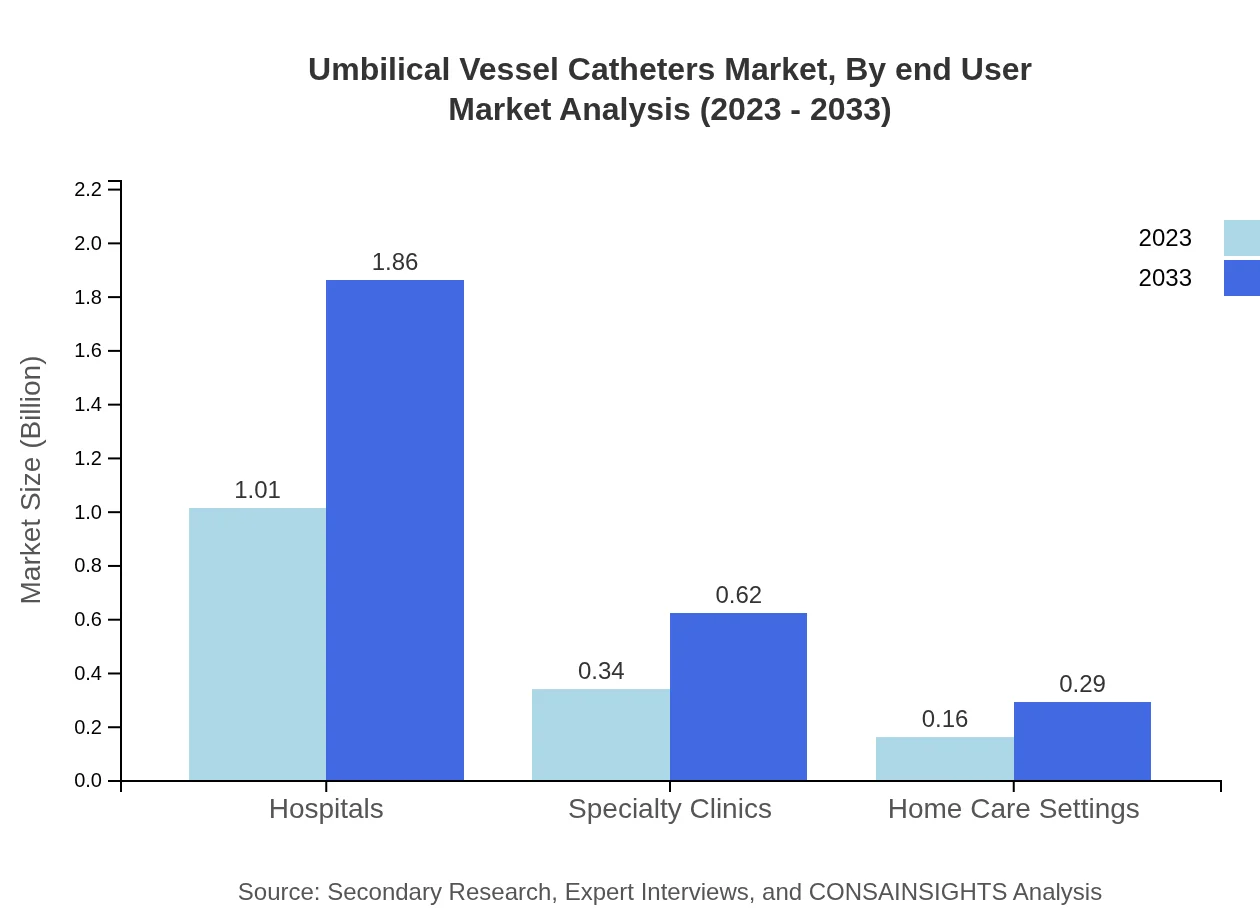
Umbilical Vessel Catheters Market Analysis By End User
The dominant segment by end-user is hospitals, with significant market size of $1.01 billion in 2023 increasing to $1.86 billion by 2033, maintaining a prevalent share of 67.02%. Specialty Clinics and Home Care Settings represent growing areas with respective market sizes of $0.34 billion and $0.16 billion, showing promising trends in adapting catheter technologies for outpatient care.
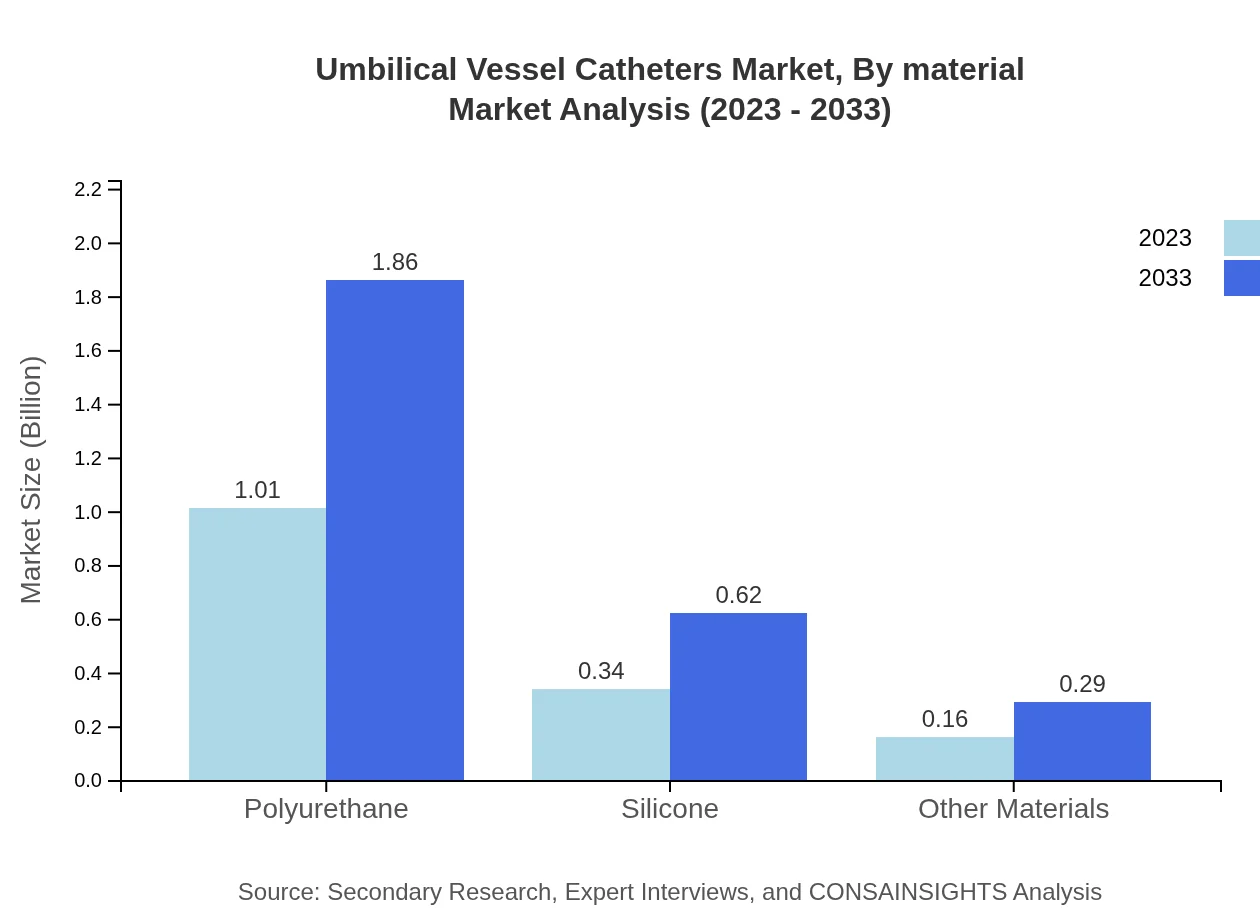
Umbilical Vessel Catheters Market Analysis By Material
The primary materials used in the production of umbilical vessel catheters include Polyurethane, Silicone, and other materials. Polyurethane shows a robust market presence with sizes of $1.01 billion for 2023 and $1.86 billion by 2033. Silicone holds a notable segment as well, projected to grow from $0.34 billion to $0.62 billion, representing 22.38% market share.
Umbilical Vessel Catheters Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Umbilical Vessel Catheters Industry
Medtronic :
A leader in medical technology, Medtronic specializes in providing innovative solutions in critical care settings, including umbilical vessel catheters.Becton, Dickinson and Company (BD):
BD is a global medical technology company renowned for its commitment to improving outcomes for medical professionals and patients via high-quality catheters.Vygon:
Vygon focuses on healthcare products, particularly for neonatal and pediatric care, offering superior catheter solutions tailored for umbilical applications.Systagenix Wound Management:
Specializes in wound and analytical products, contributing to innovations in umbilical catheters to enhance safety and control.We're grateful to work with incredible clients.









FAQs
What is the market size of umbilical vessel catheters?
The global market size for umbilical vessel catheters is projected to reach $1.5 billion by 2033, growing at a CAGR of 6.2% from 2023 to 2033, driven by increasing neonatal healthcare needs.
What are the key market players or companies in this umbilical vessel catheters industry?
Key market players in the umbilical vessel catheters industry include major healthcare companies and medical device manufacturers focusing on neonatal care products, innovation, and distribution.
What are the primary factors driving the growth in the umbilical vessel catheters industry?
Growth in the umbilical vessel catheters market is driven by rising premature births, advancements in neonatal care technologies, and increasing investment in healthcare infrastructure globally.
Which region is the fastest Growing in the umbilical vessel catheters?
The fastest-growing region for umbilical vessel catheters is North America, expected to grow from $0.48 billion in 2023 to $0.89 billion by 2033, reflecting enhanced neonatal healthcare initiatives.
Does ConsaInsights provide customized market report data for the umbilical vessel catheters industry?
Yes, ConsaInsights provides customized market report data for the umbilical vessel catheters industry, tailored to specific client needs including segment analysis and trend forecasts.
What deliverables can I expect from this umbilical vessel catheters market research project?
Deliverables from the umbilical vessel catheters market research project include detailed market analysis, trend reports, segmented data insights, competitive landscape, and strategic recommendations.
What are the market trends of umbilical vessel catheters?
Current market trends in umbilical vessel catheters include a focus on innovative materials like polyurethane, increased usage in neonatal intensive care, and a rise in home care settings.