Ureteral Stent Market Report
Published Date: 31 January 2026 | Report Code: ureteral-stent
Ureteral Stent Market Size, Share, Industry Trends and Forecast to 2033
This report covers the comprehensive analysis of the Ureteral Stent market, encompassing market size, growth forecasts, key trends, and regional insights from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
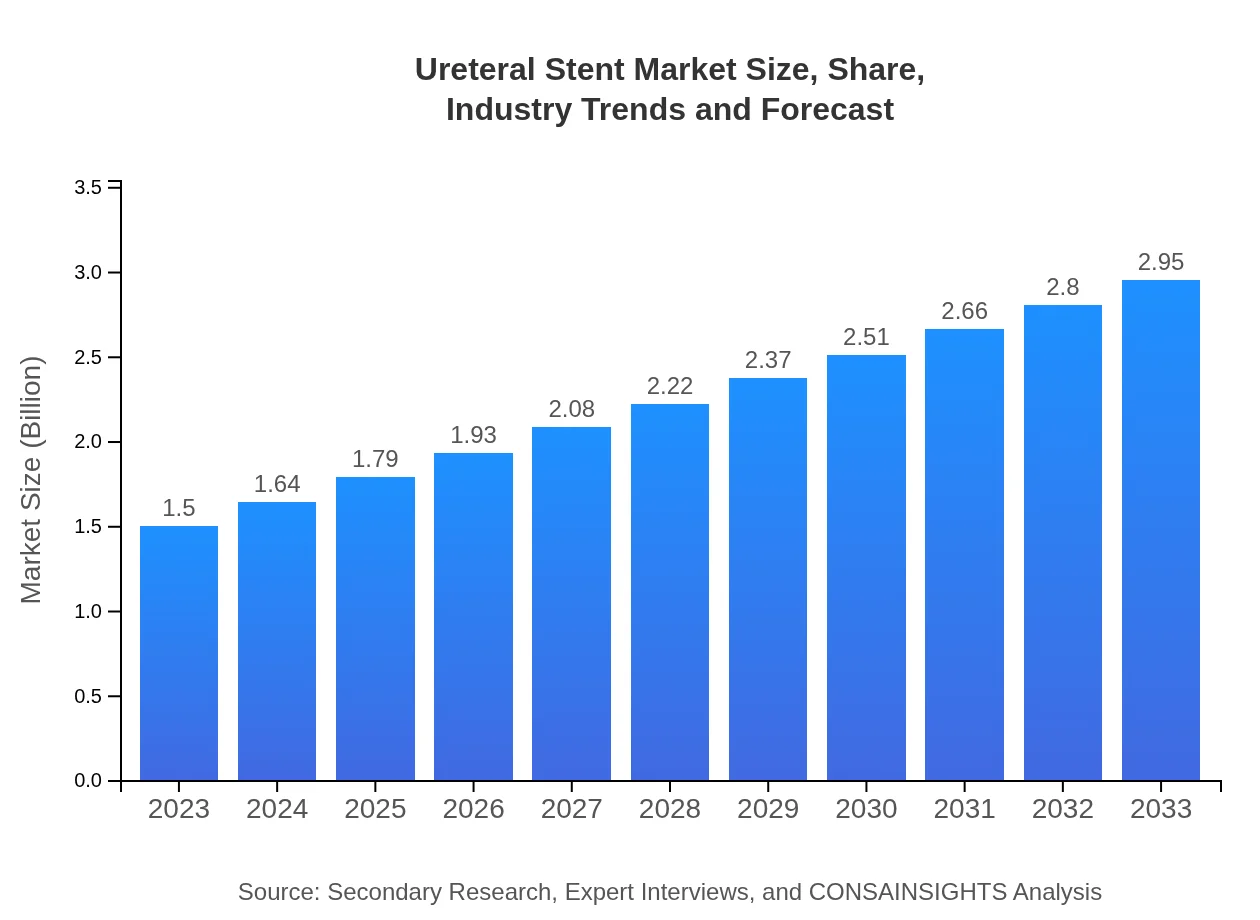
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | Boston Scientific Corporation, Bard Medical, Cook Medical, Coloplast A/S |
| Last Modified Date | 31 January 2026 |
Ureteral Stent Market Overview
Customize Ureteral Stent Market Report market research report
- ✔ Get in-depth analysis of Ureteral Stent market size, growth, and forecasts.
- ✔ Understand Ureteral Stent's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Ureteral Stent
What is the Market Size & CAGR of Ureteral Stent market in 2023?
Ureteral Stent Industry Analysis
Ureteral Stent Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Ureteral Stent Market Analysis Report by Region
Europe Ureteral Stent Market Report:
The European market's size in 2023 stands at $0.42 billion, projected to grow to $0.83 billion by 2033. This growth can be attributed to strong healthcare reimbursement policies and increasing awareness of urinary tract health.Asia Pacific Ureteral Stent Market Report:
In the Asia Pacific region, the Ureteral Stent market was valued at $0.28 billion in 2023 and is projected to grow to $0.56 billion by 2033. Factors influencing this growth include a growing aging population and increasing prevalence of urological disorders, along with improving access to healthcare facilities.North America Ureteral Stent Market Report:
North America boasts the largest market size, with $0.58 billion in 2023 and an anticipated increase to $1.14 billion by 2033. Factors include high healthcare spending, advanced healthcare infrastructure, and significant demand for innovative stent technologies.South America Ureteral Stent Market Report:
For South America, the market size is currently at $0.08 billion in 2023, with expectations to rise to $0.15 billion by 2033. The growth is driven by increasing healthcare investments and advancements in medical technologies across the region.Middle East & Africa Ureteral Stent Market Report:
In the Middle East and Africa, the market is currently valued at $0.13 billion, with future estimates of $0.26 billion by 2033. Factors like rising incidence of kidney stones and expanding healthcare expenditure contribute to the anticipated growth.Tell us your focus area and get a customized research report.
Ureteral Stent Market Analysis By Product Type
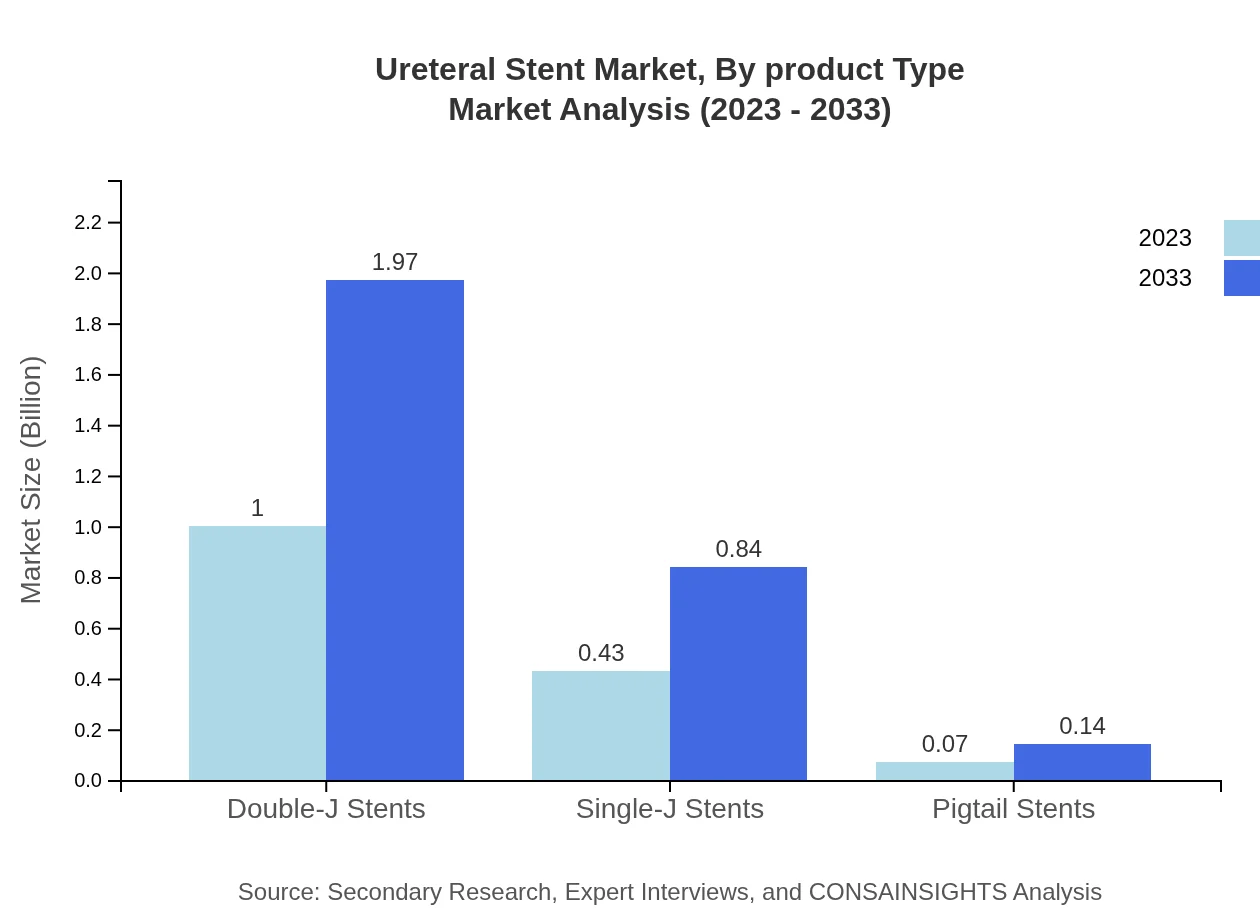
The Ureteral Stent market, segmented by product type, shows double-J stents dominating with a market size of $1.00 billion in 2023, projected to reach $1.97 billion by 2033. Single-J and pigtail stents also play significant roles, focusing on various urological applications. Their uses are crucial in procedures like rigid or flexible cystoscopy transformations.
Ureteral Stent Market Analysis By Material
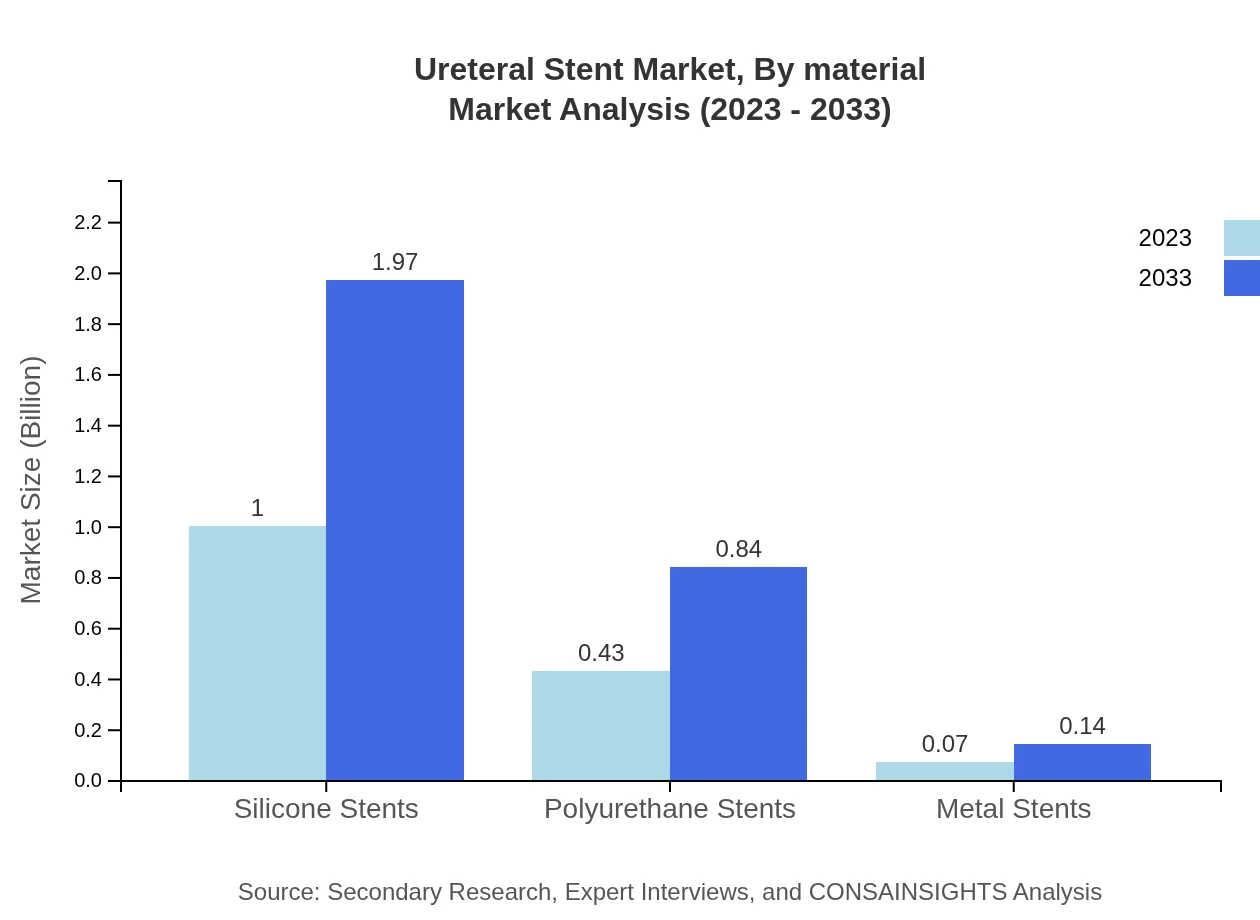
Silicone stents lead the material segment with a market share of 66.76%, valued at $1.00 billion in 2023, increasing at a steady pace to $1.97 billion by 2033. Polyurethane stents and metal stents are capturing niche applications, demonstrating their unique benefits, such as durability and biocompatibility.
Ureteral Stent Market Analysis By Application
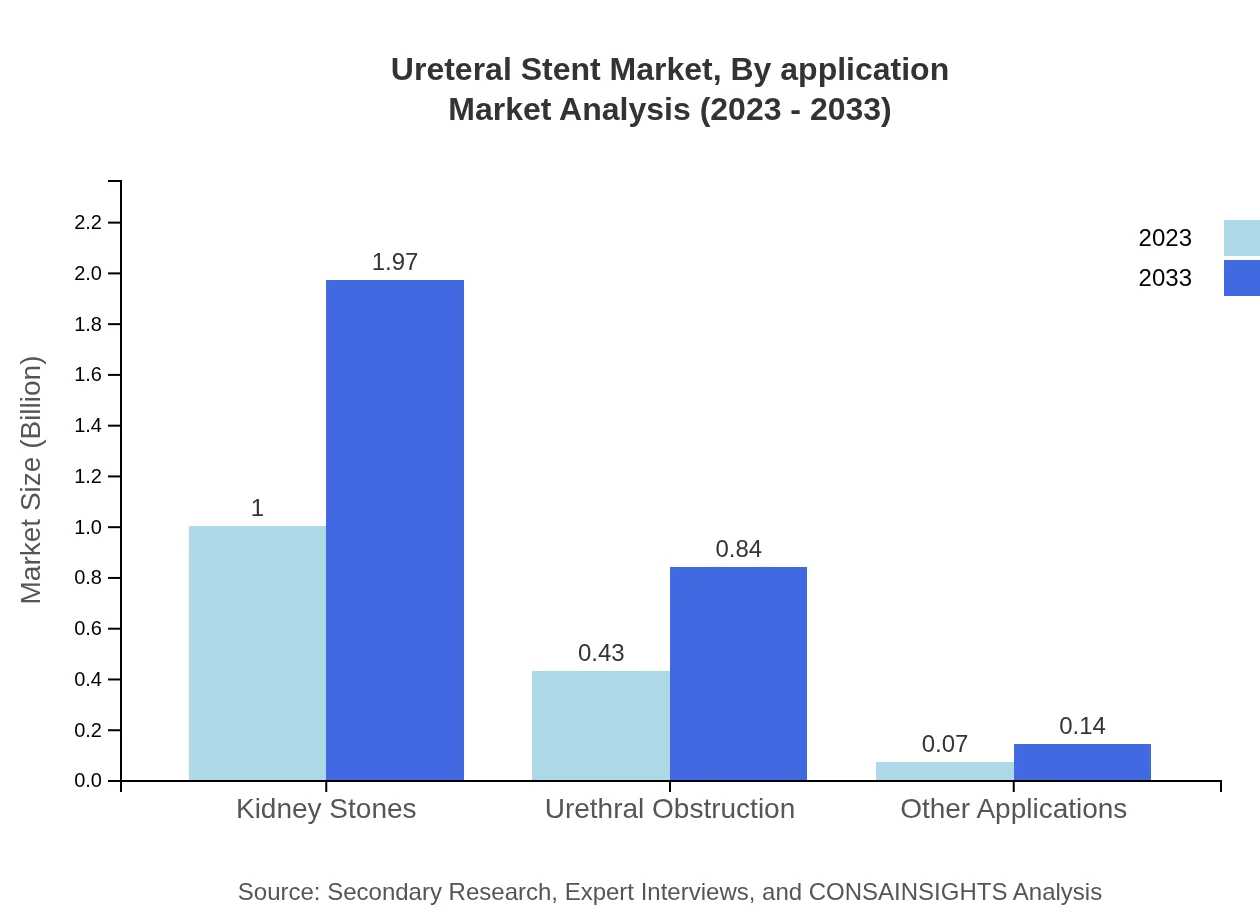
The primary application area for ureteral stents is kidney stones, representing a compelling market share of 66.76%. Urethral obstruction follows closely, covering critical concerns in urology. The market is witnessing a surge in innovations that respond to these specific applications.
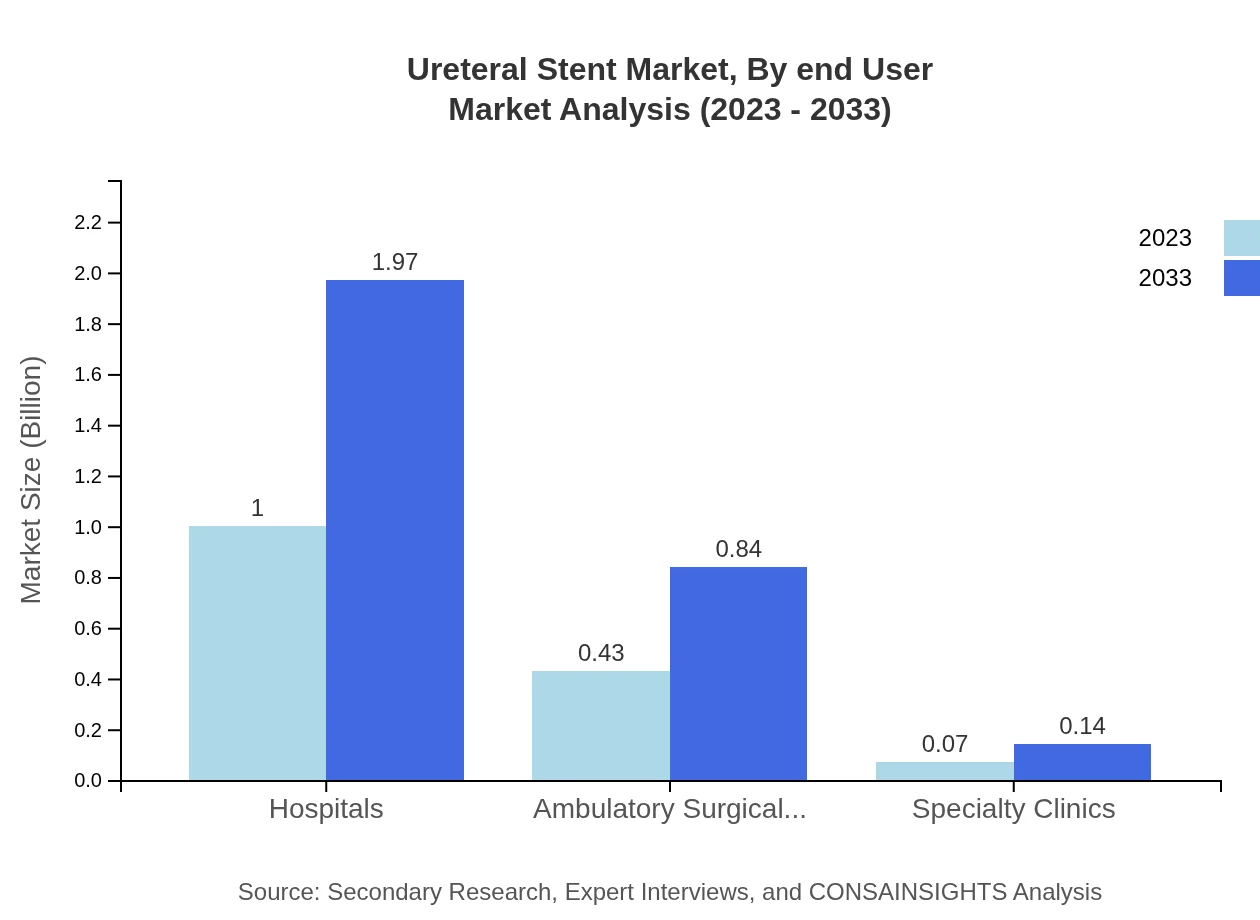
Ureteral Stent Market Analysis By End User
Hospitals remain the primary end-users of ureteral stents, accounting for 66.76% market share with a projected growth from $1.00 billion in 2023 to $1.97 billion in 2033. Ambulatory surgical centers and specialty clinics are significant contributors as well.
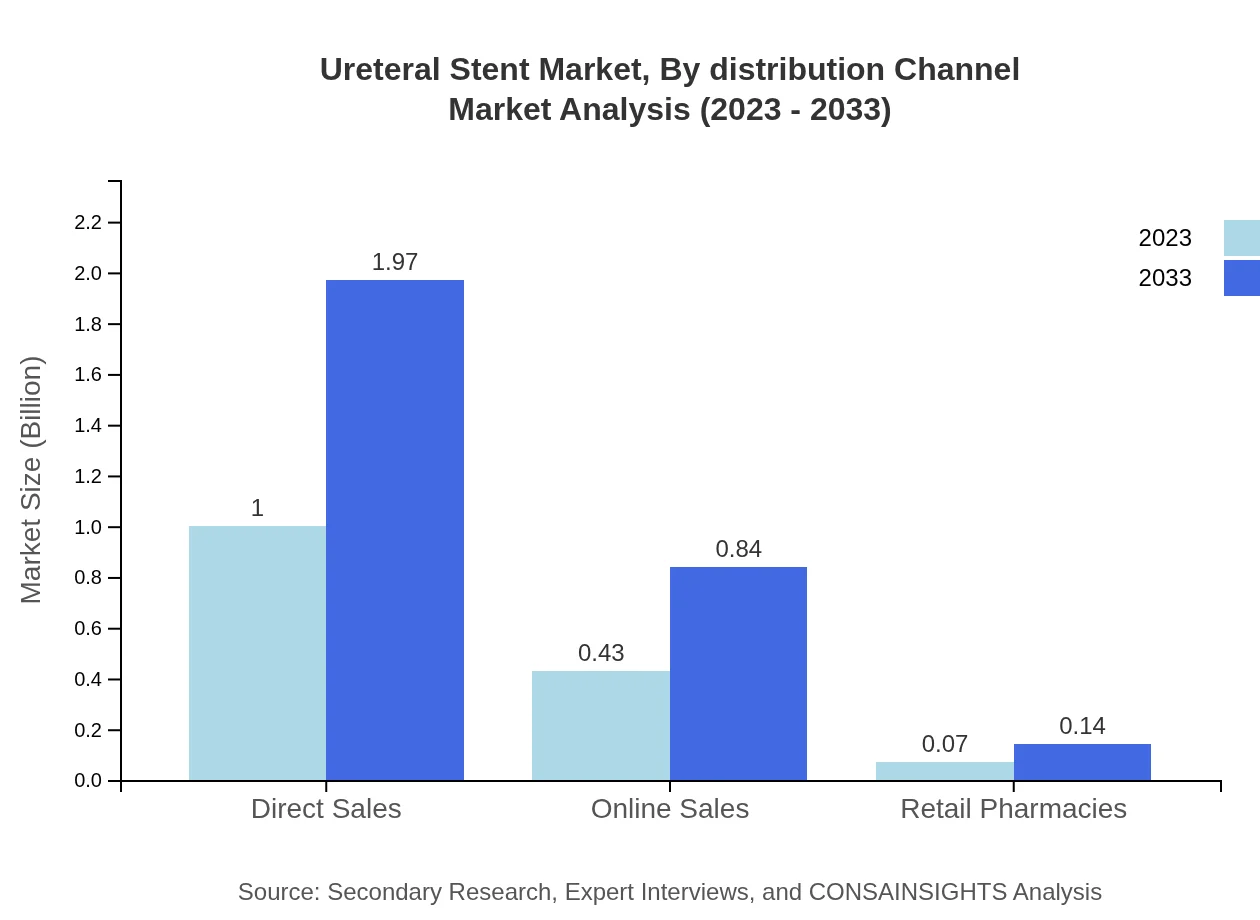
Ureteral Stent Market Analysis By Distribution Channel
Direct sales dominate the distribution channel segment with a steady share of 66.76%. However, online sales are gaining traction, indicative of the rising trend of e-commerce in medical device distribution, supporting wider patient access.
Ureteral Stent Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Ureteral Stent Industry
Boston Scientific Corporation:
A leader in the medical device field, Boston Scientific specializes in innovative urology solutions, including advanced ureteral stent technologies to enhance patient care.Bard Medical:
A subsidiary of Becton, Dickinson and Company, Bard Medical focuses on stents and other urological products, renowned for high-quality devices and extensive market reach.Cook Medical:
Cook Medical is known for its broad array of ureteral stent products, dedicated to advancing patient outcomes through innovative medical technologies.Coloplast A/S:
Specializing in urology and continence care, Coloplast provides a range of gravitation and supportive stents designed to improve patient quality of life.We're grateful to work with incredible clients.









FAQs
What is the market size of the ureteral Stent?
The global ureteral stent market is valued at approximately 1.5 billion in 2023 with a projected growth at a CAGR of 6.8% by 2033. This growth indicates significant demand within the healthcare sector for ureteral stents in various applications.
What are the key market players or companies in this ureteral Stent industry?
Key players in the ureteral stent market include major medical device manufacturers such as Boston Scientific, Coloplast, and Cook Medical. These companies are pivotal in advancing products and expanding market reach through innovation and strategic partnerships.
What are the primary factors driving the growth in the ureteral stent industry?
Factors driving growth include the rising prevalence of kidney stones and other urological conditions, advancements in medical technology, and an increasing number of urological procedures. Additionally, an aging population and increased awareness contribute to market expansion.
Which region is the fastest Growing in the ureteral stent?
North America is anticipated to be the fastest-growing region, with a market value of 0.58 billion in 2023 projected to reach 1.14 billion by 2033. Europe and Asia Pacific also show significant growth potential in the ureteral stent market during this period.
Does ConsaInsights provide customized market report data for the ureteral stent industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the ureteral stent industry. This includes detailed insights and analyses to help stakeholders make informed decisions based on market trends and dynamics.
What deliverables can I expect from this ureteral stent market research project?
Deliverables from the ureteral stent market research project typically include comprehensive reports, detailed market analysis, forecasts, competitive landscape assessments, and insights on key trends and growth drivers within the industry.
What are the market trends of ureteral stent?
Current market trends for ureteral stents include increased adoption of minimally invasive procedures, growing demand for biodegradable stents, and significant advancements in materials used for stent production, which improve patient outcomes and reduce complications.