Urothelial Cancer Drugs Market Report
Published Date: 31 January 2026 | Report Code: urothelial-cancer-drugs
Urothelial Cancer Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report analyzes the Urothelial Cancer Drugs market, providing insights into market dynamics, size, segmentation, and regional trends. The forecast period spans from 2023 to 2033, offering a comprehensive assessment for stakeholders in the oncology sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
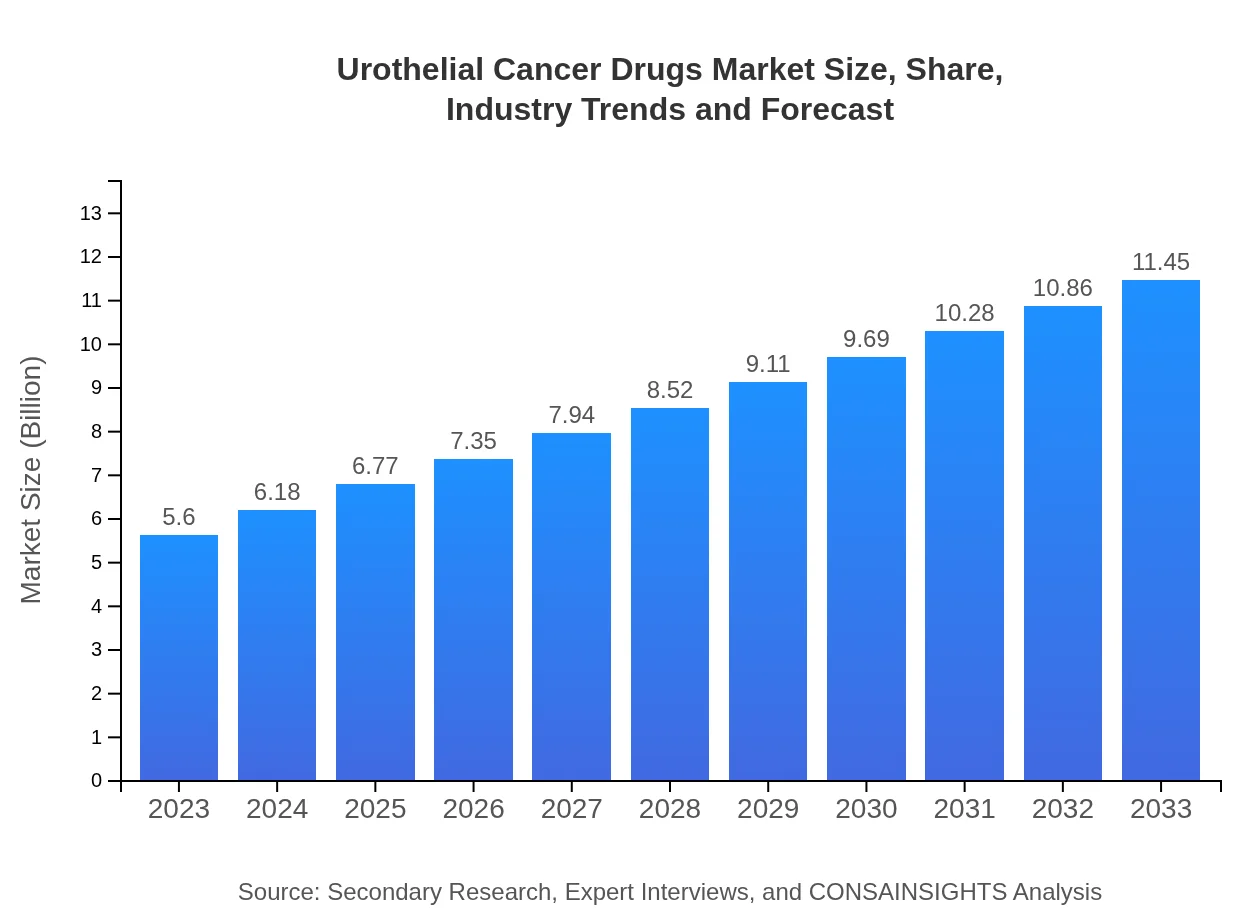
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | AstraZeneca, Roche, Merck & Co., Bristol Myers Squibb |
| Last Modified Date | 31 January 2026 |
Urothelial Cancer Drugs Market Overview
Customize Urothelial Cancer Drugs Market Report market research report
- ✔ Get in-depth analysis of Urothelial Cancer Drugs market size, growth, and forecasts.
- ✔ Understand Urothelial Cancer Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Urothelial Cancer Drugs
What is the Market Size & CAGR of Urothelial Cancer Drugs market in 2023?
Urothelial Cancer Drugs Industry Analysis
Urothelial Cancer Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Urothelial Cancer Drugs Market Analysis Report by Region
Europe Urothelial Cancer Drugs Market Report:
European market size is set to rise from $1.53 billion in 2023 to $3.13 billion by 2033, supported by innovative drug approvals and increasing prevalence of bladder cancer that necessitate effective treatments.Asia Pacific Urothelial Cancer Drugs Market Report:
In the Asia Pacific region, the Urothelial Cancer Drugs market is expected to witness growth from $1.09 billion in 2023 to $2.23 billion by 2033, fueled by increasing healthcare expenditure and rising cancer awareness.North America Urothelial Cancer Drugs Market Report:
North America dominates the Urothelial Cancer Drugs market, projected to expand from $2.14 billion in 2023 to $4.37 billion by 2033. The region's advanced healthcare infrastructure and significant R&D investments are key growth drivers.South America Urothelial Cancer Drugs Market Report:
The South American market is estimated to grow from $0.41 billion in 2023 to $0.84 billion by 2033. A focus on improving healthcare infrastructure and access to newer therapies will drive market expansion.Middle East & Africa Urothelial Cancer Drugs Market Report:
The market in the Middle East and Africa is projected to grow from $0.44 billion in 2023 to $0.89 billion by 2033. Factors such as improving healthcare systems and awareness campaigns are expected to enhance drug accessibility.Tell us your focus area and get a customized research report.
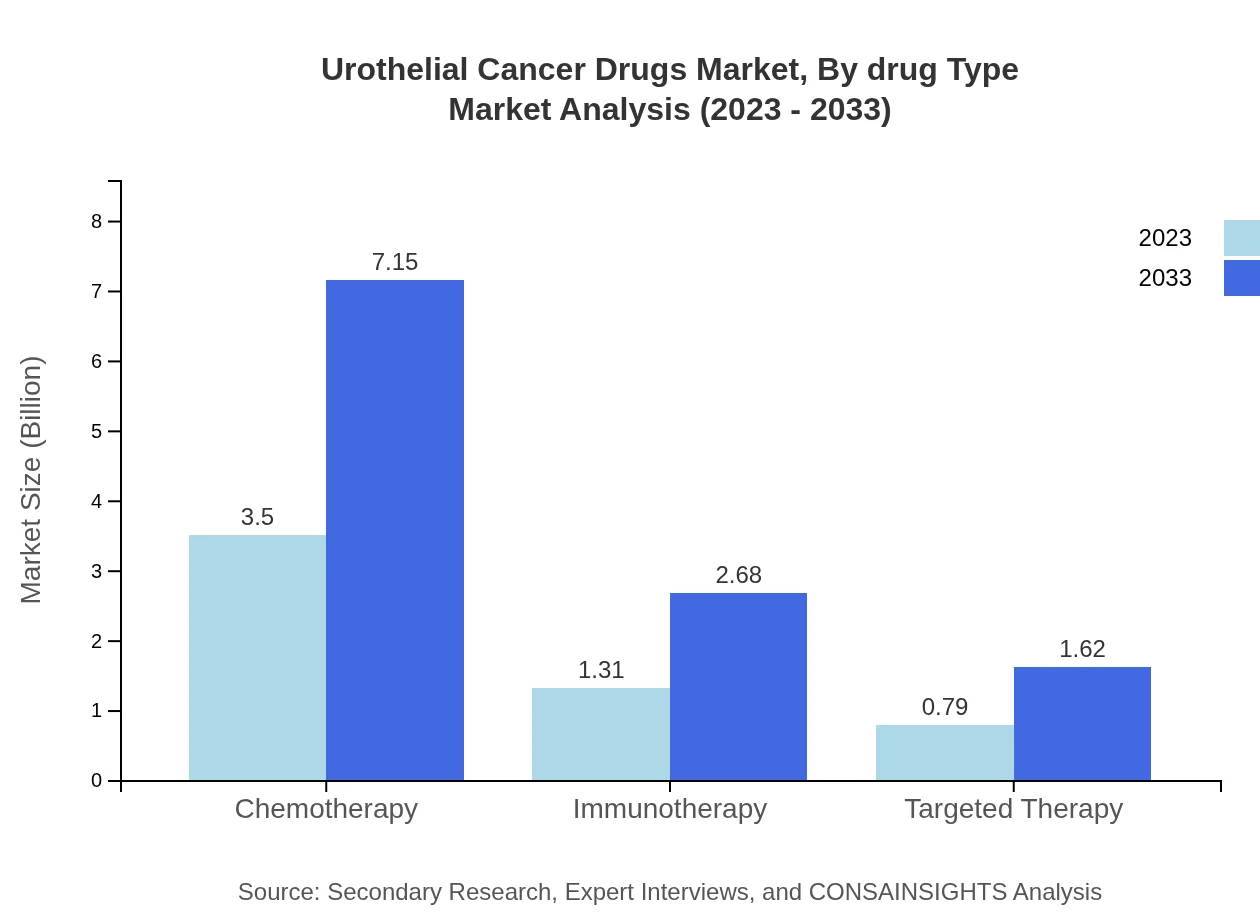
Urothelial Cancer Drugs Market Analysis By Drug Type
In 2023, chemotherapy accounts for a significant $3.50 billion and remains the leader with a market share of 62.47%. Immunotherapy follows with $1.31 billion (23.38%), while targeted therapy captures $0.79 billion (14.15%). By 2033, the values are projected to reach $7.15 billion, $2.68 billion, and $1.62 billion respectively, indicating a shift towards targeted and immunotherapeutic options.
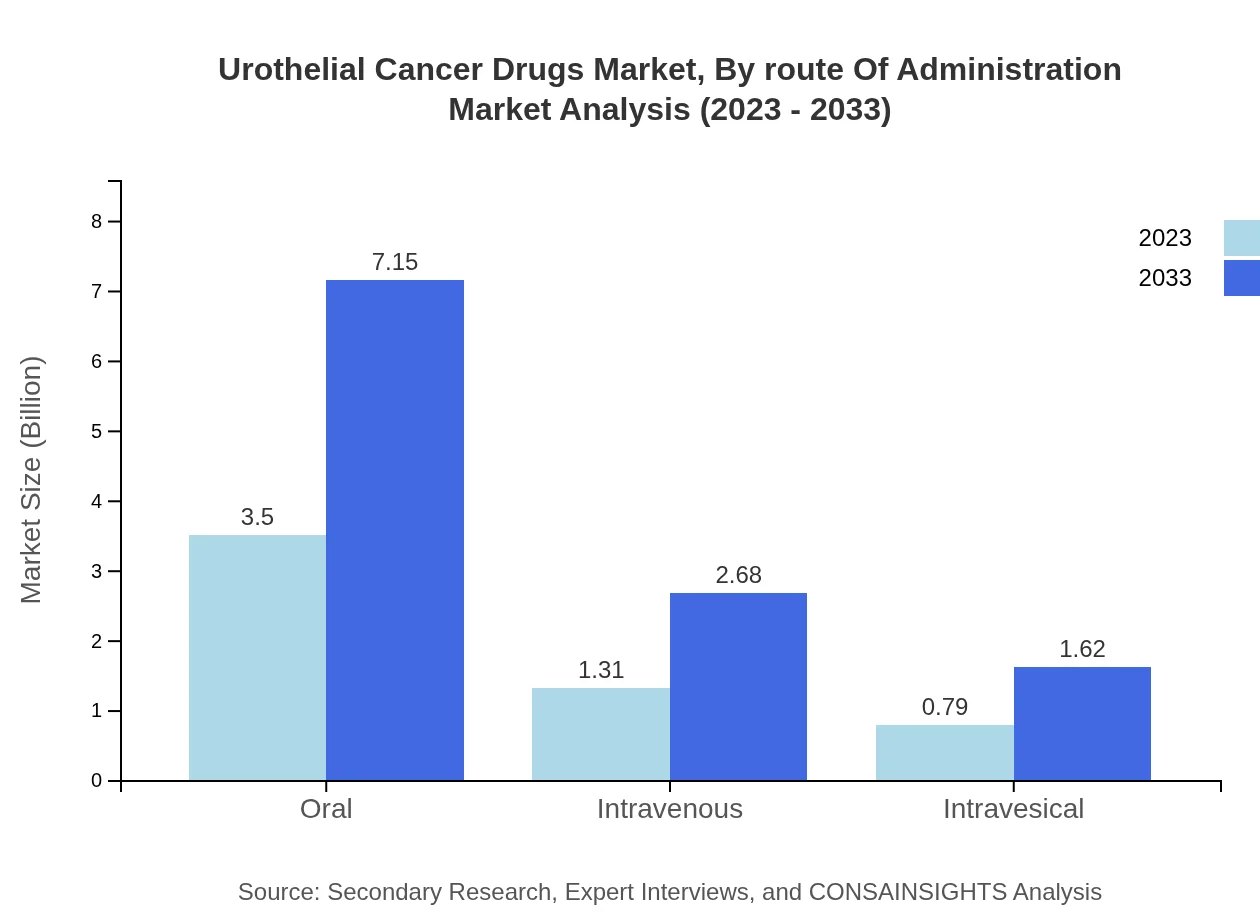
Urothelial Cancer Drugs Market Analysis By Route Of Administration
The oral administration route leads the segment in 2023 with a market size of $3.50 billion (62.47%) and is expected to double in value by 2033 to $7.15 billion. Intravenous routes account for $1.31 billion in 2023 with a market share of 23.38%, while intravesical delivery options represent $0.79 billion (14.15%). This trend reflects an increasing preference for oral therapies due to their ease of use.
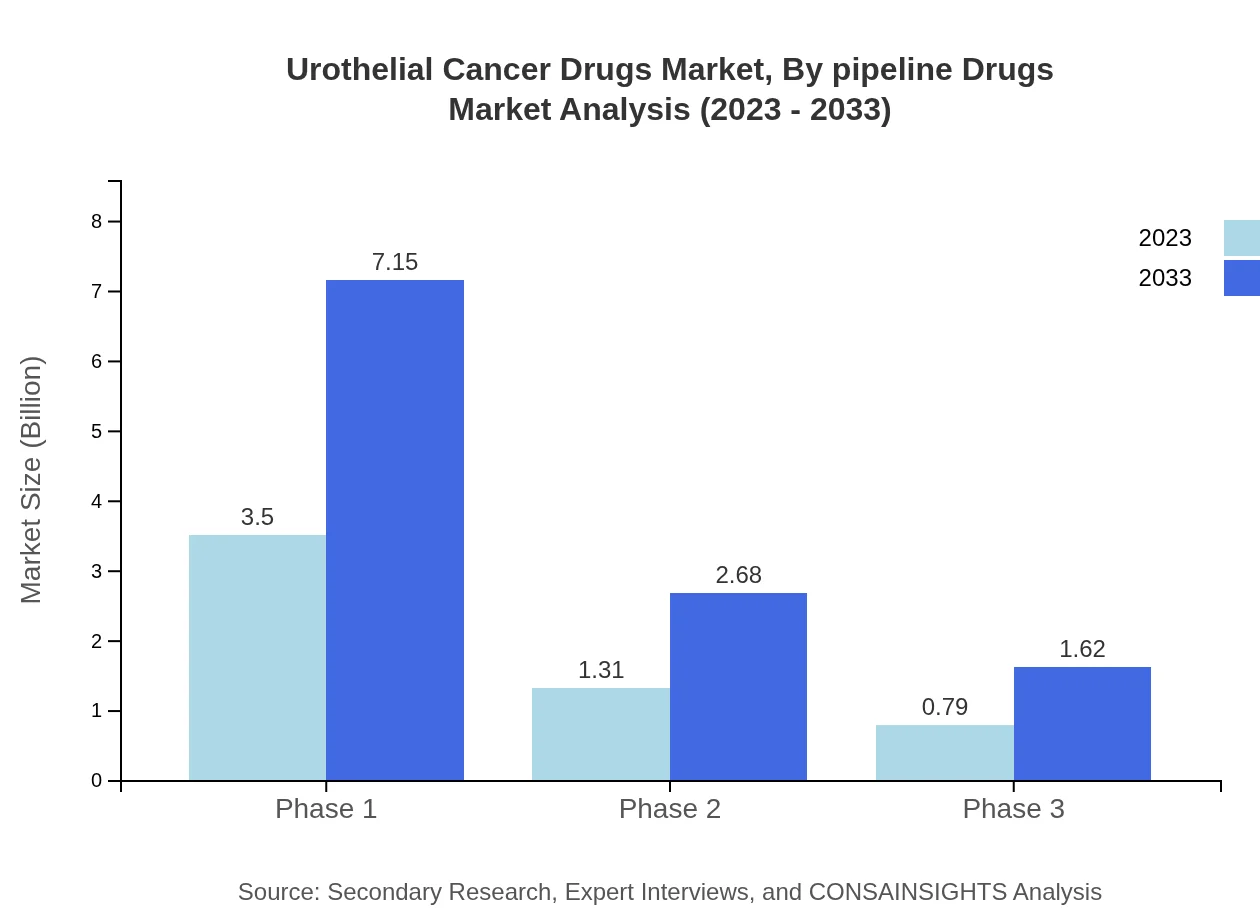
Urothelial Cancer Drugs Market Analysis By Pipeline Drugs
Pipeline drugs targeting novel mechanisms of action show promising potential. As of 2023, ongoing trials for Phase 1, Phase 2, and Phase 3 drugs are at market sizes of $3.50 billion (62.47%), $1.31 billion (23.38%), and $0.79 billion (14.15%) respectively. By 2033, these are forecasted to grow to $7.15 billion, $2.68 billion, and $1.62 billion, emphasizing the robust R&D efforts in developing next-generation therapies.
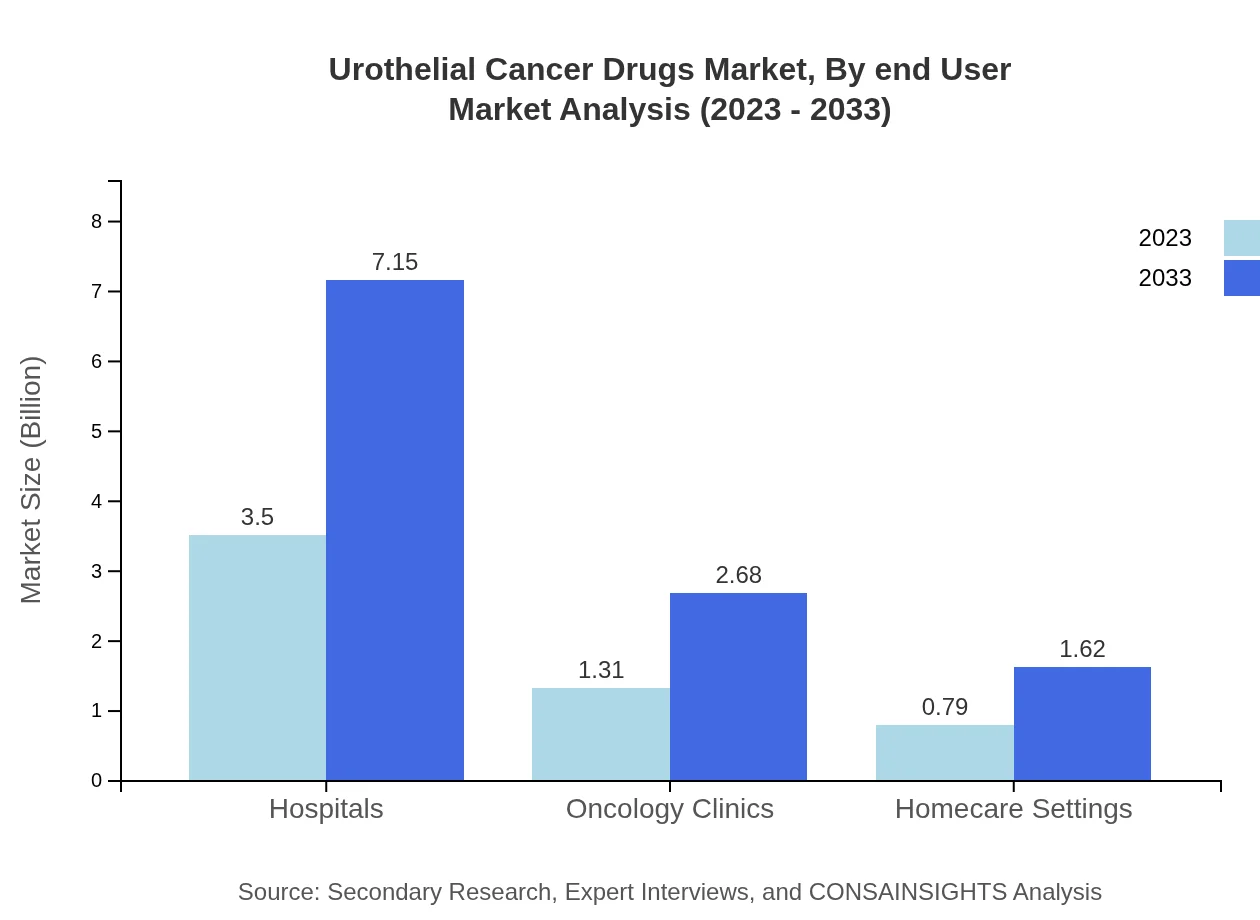
Urothelial Cancer Drugs Market Analysis By End User
Hospitals dominate the end-user segment, accounting for $3.50 billion (62.47%) in 2023 and expected to grow to $7.15 billion by 2033. Oncology clinics follow with $1.31 billion (23.38%) and are projected to reach $2.68 billion. Homecare settings, currently at $0.79 billion (14.15%), are emerging as a significant channel reflecting patient preference for at-home treatment options.
Urothelial Cancer Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Urothelial Cancer Drugs Industry
AstraZeneca:
AstraZeneca is a leading player in the Urothelial Cancer Drugs market, recognized for its groundbreaking immunotherapy, Atezolizumab, which has transformed treatment protocols for advanced bladder cancer.Roche:
Roche develops innovative treatments for bladder cancer, notably transitioning towards personalized medicine that tailors therapy based on genetic profiles of tumors.Merck & Co.:
Merck & Co. is notable for its comprehensive oncology portfolio, including therapies aimed at managing urothelial carcinoma and contributing significantly to clinical research.Bristol Myers Squibb:
Bristol Myers Squibb plays a vital role in the development of targeted therapies for urothelial cancer, focusing on enhancing patient outcomes through innovative solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of urothelial cancer drugs?
The urothelial cancer drugs market size is projected at $5.6 billion in 2023, with a CAGR of 7.2% expected until 2033. This growth indicates significant advancements in treatment options and increasing patient populations requiring effective therapies.
What are the key market players or companies in the urothelial cancer drugs industry?
Key players in the urothelial cancer drugs market include large pharmaceutical companies and biotechnology firms focusing on innovative cancer therapies. Their ongoing research and development efforts contribute significantly to the overall market growth, addressing unmet medical needs.
What are the primary factors driving the growth in the urothelial cancer drugs industry?
Key growth drivers in the urothelial cancer drugs market include increasing cancer incidence rates, advancements in treatment modalities like immunotherapy, and rising investments in oncology research. Additionally, early diagnosis and improved treatment outcomes play crucial roles.
Which region is the fastest Growing in the urothelial cancer drugs?
North America is identified as the fastest-growing region for urothelial cancer drugs, with a market size projected to increase from $2.14 billion in 2023 to $4.37 billion by 2033, supported by advanced healthcare infrastructure and significant R&D investments.
Does ConsaInsights provide customized market report data for the urothelial cancer drugs industry?
Yes, ConsaInsights offers tailored market reports for the urothelial cancer drugs industry, catering to specific client needs. These custom reports deliver in-depth insights, competitive analysis, and market forecasts tailored to individual business objectives.
What deliverables can I expect from this urothelial cancer drugs market research project?
Expect comprehensive deliverables including market analysis, competitor profiles, trend assessments, and future predictions. Detailed segmentation and regional insights ensure actionability for strategic planning and investment decisions.
What are the market trends of urothelial cancer drugs?
Current trends in the urothelial cancer drugs market indicate a shift towards personalized medicine, increased use of combination therapies, and a significant focus on immunooncology. These trends aim to enhance treatment efficacy and improve patient outcomes.