Uterine Cancer Therapeutics Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: uterine-cancer-therapeutics-diagnostics
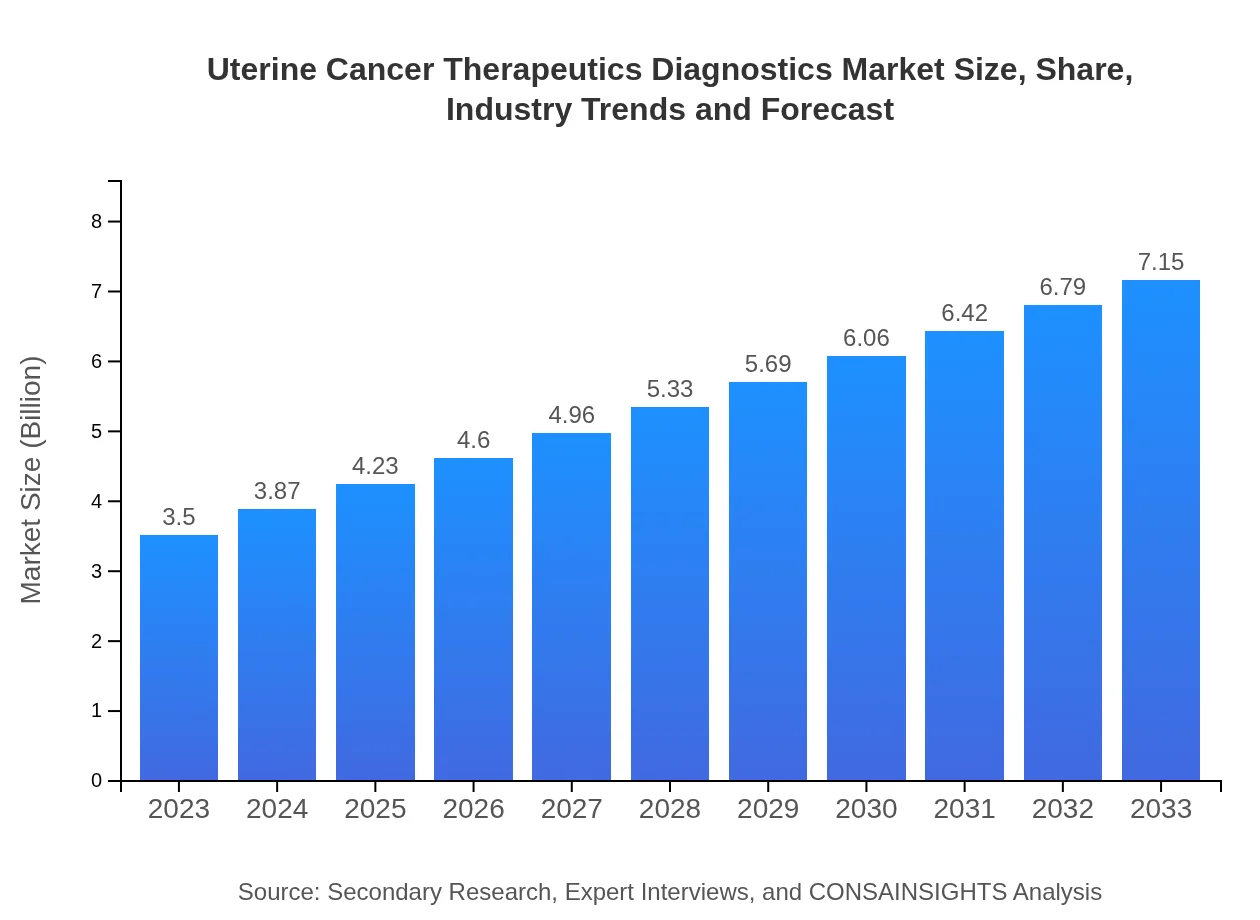
Uterine Cancer Therapeutics Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Uterine Cancer Therapeutics Diagnostics market from 2023 to 2033, emphasizing market trends, sizes, and growth forecasts. It highlights essential insights into regional dynamics, industry analysis, segmentation, and key players shaping the future of therapeutic diagnostics in this sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $7.15 Billion |
| Top Companies | Roche, Merck & Co., Bristol Myers Squibb, Novartis, AbbVie |
| Last Modified Date | 31 January 2026 |
Uterine Cancer Therapeutics Diagnostics Market Overview
Customize Uterine Cancer Therapeutics Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Uterine Cancer Therapeutics Diagnostics market size, growth, and forecasts.
- ✔ Understand Uterine Cancer Therapeutics Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Uterine Cancer Therapeutics Diagnostics
What is the Market Size & CAGR of Uterine Cancer Therapeutics Diagnostics market in 2033?
Uterine Cancer Therapeutics Diagnostics Industry Analysis
Uterine Cancer Therapeutics Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Uterine Cancer Therapeutics Diagnostics Market Analysis Report by Region
Europe Uterine Cancer Therapeutics Diagnostics Market Report:
The European market is also expected to exhibit significant growth, expanding from USD 1.04 billion in 2023 to USD 2.12 billion by 2033. Increased awareness of uterine cancer, along with robust healthcare policies that promote cancer screening and treatment, are prominent growth factors.Asia Pacific Uterine Cancer Therapeutics Diagnostics Market Report:
The Asia Pacific region is witnessing a rapid increase in the Uterine Cancer Therapeutics Diagnostics market, expected to grow from USD 0.63 billion in 2023 to USD 1.29 billion by 2033. This growth can be attributed to improving healthcare infrastructure, increased access to advanced therapeutic options, and rising government initiatives aimed at cancer awareness and prevention.North America Uterine Cancer Therapeutics Diagnostics Market Report:
North America dominates the Uterine Cancer Therapeutics Diagnostics market, with significant growth expected from USD 1.31 billion in 2023 to USD 2.68 billion by 2033. The region benefits from advanced healthcare facilities, high adoption rates of new technologies, and substantial funding for cancer research.South America Uterine Cancer Therapeutics Diagnostics Market Report:
In South America, the market is projected to grow mildly from USD 0.09 billion in 2023 to USD 0.18 billion by 2033. The growth is significantly influenced by increasing healthcare investments and improved access to diagnostics, which aim to enhance early detection rates of uterine cancer.Middle East & Africa Uterine Cancer Therapeutics Diagnostics Market Report:
In the Middle East and Africa, the market is anticipated to grow from USD 0.44 billion in 2023 to USD 0.89 billion by 2033, driven by improving healthcare systems and ongoing strategic initiatives aimed at cancer prevention and management.Tell us your focus area and get a customized research report.
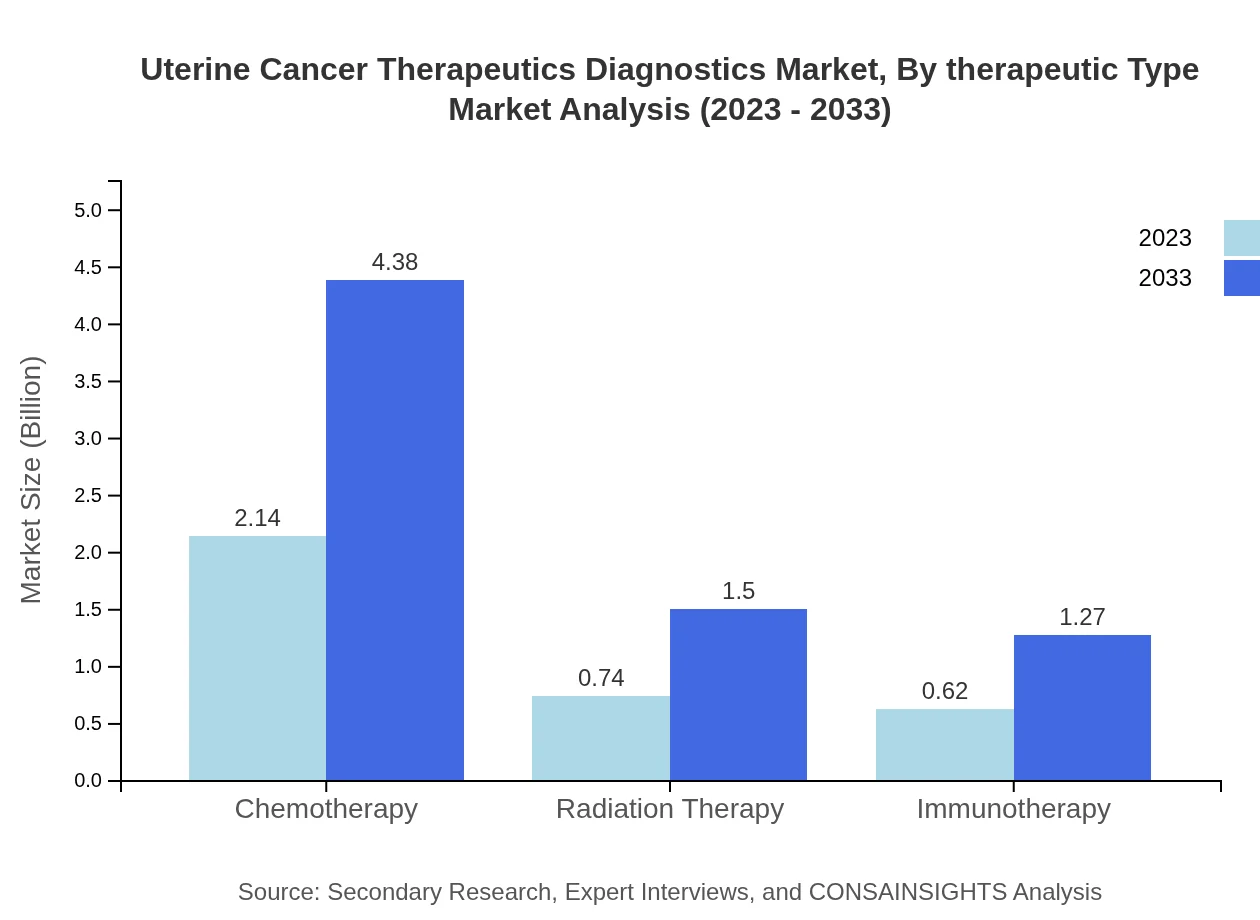
Uterine Cancer Therapeutics Diagnostics Market Analysis By Therapeutic Type
The market for therapeutics is characterized by significant contributions from antineoplastics, which account for approximately USD 2.14 billion in 2023 and are expected to reach USD 4.38 billion by 2033. Hormone therapy and targeted therapy segments have shown steady growth, reflecting a trend towards personalized treatment approaches.
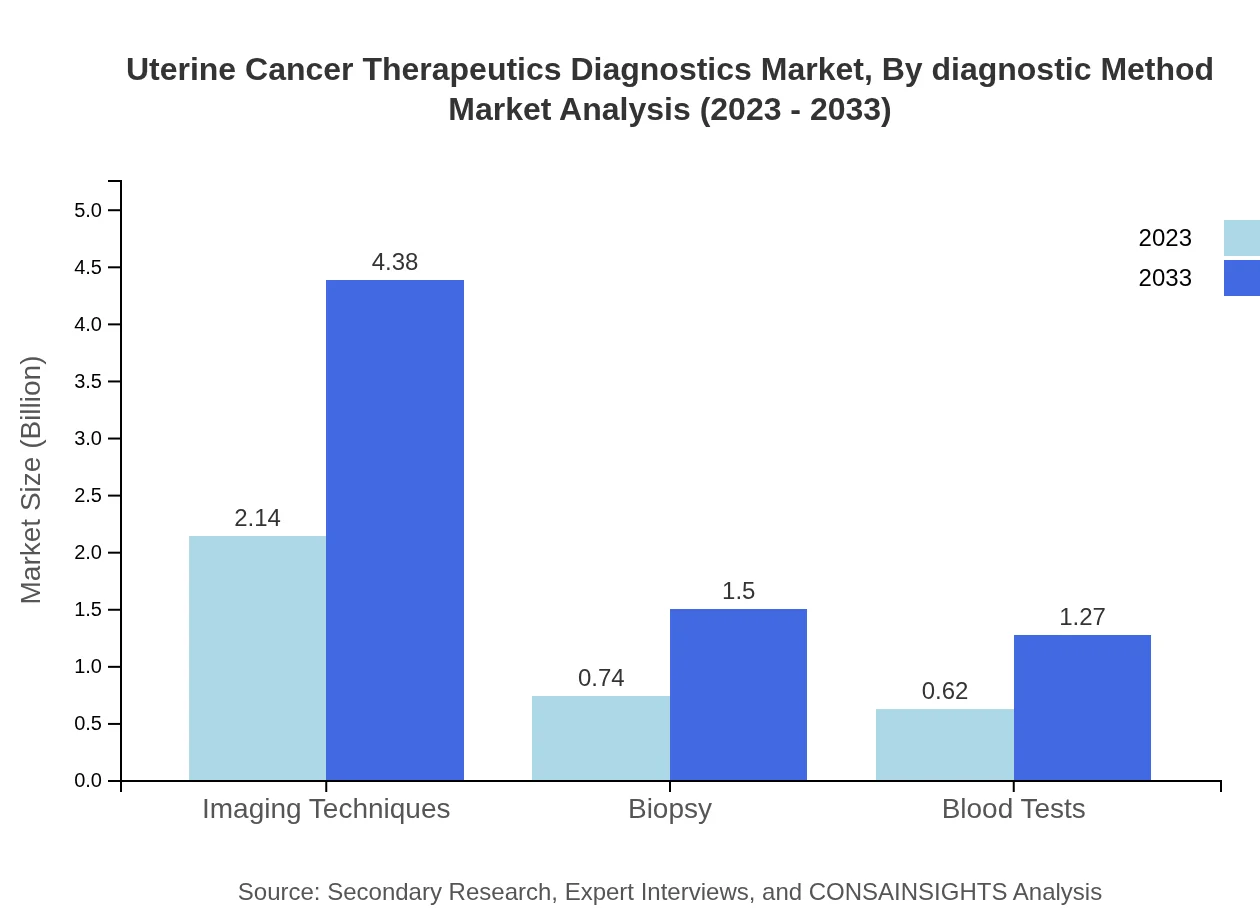
Uterine Cancer Therapeutics Diagnostics Market Analysis By Diagnostic Method
In terms of diagnostic methods, imaging techniques lead the market significantly, with a size of USD 2.14 billion in 2023 projected to grow to USD 4.38 billion by 2033. Other methods such as biopsies and blood tests also play critical roles, contributing to accurate disease diagnosis and effective treatment plans.
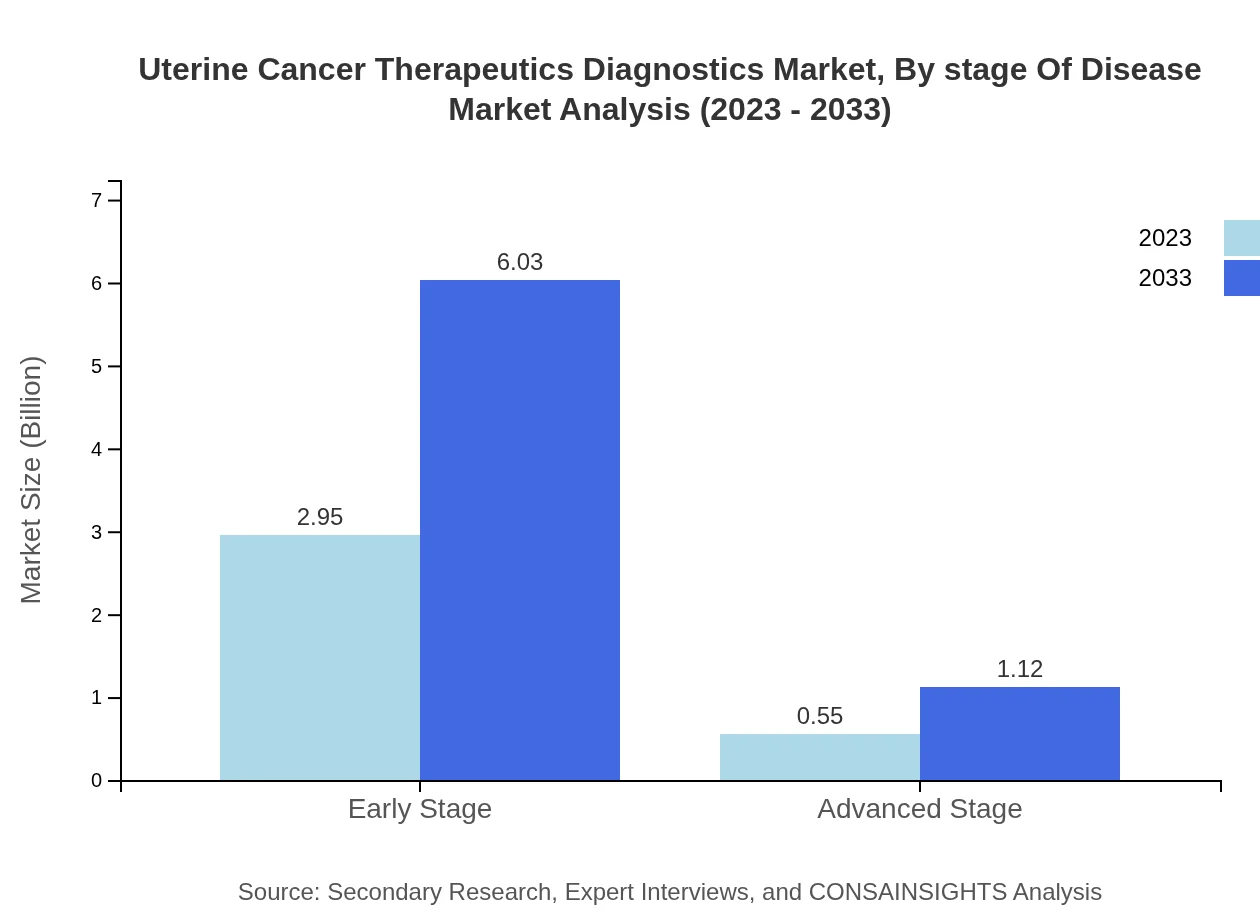
Uterine Cancer Therapeutics Diagnostics Market Analysis By Stage Of Disease
The early-stage disease segment represents a significant portion of the market, reaching USD 2.95 billion in 2023 and anticipated to rise to USD 6.03 billion by 2033, demonstrating the focus on early detection in therapeutic strategies.
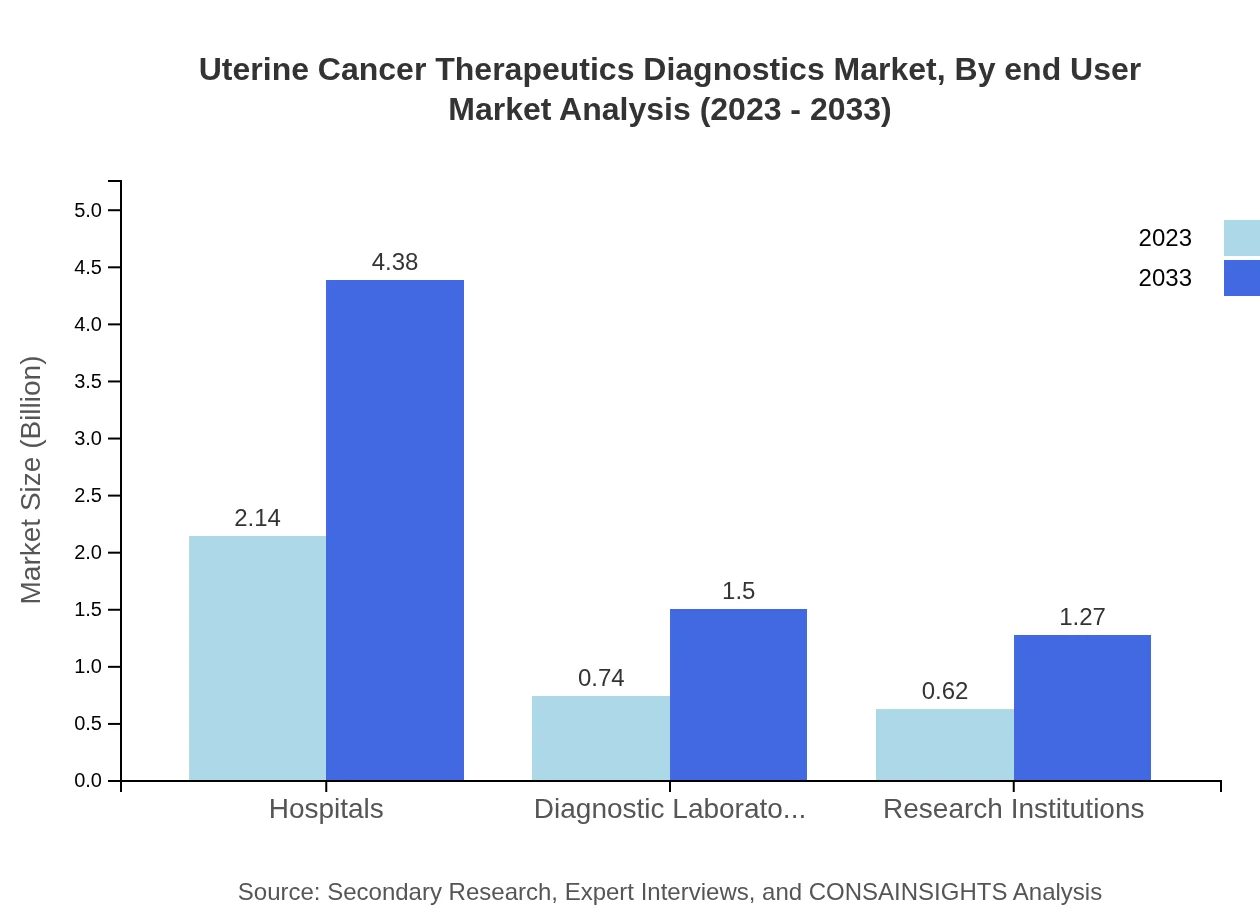
Uterine Cancer Therapeutics Diagnostics Market Analysis By End User
Hospitals remain the primary end-users in the Uterine Cancer Therapeutics Diagnostics market, accounting for 61.2% market share and poised for growth given their pivotal role in handling complex cancer cases requiring comprehensive care and advanced diagnostic measures.
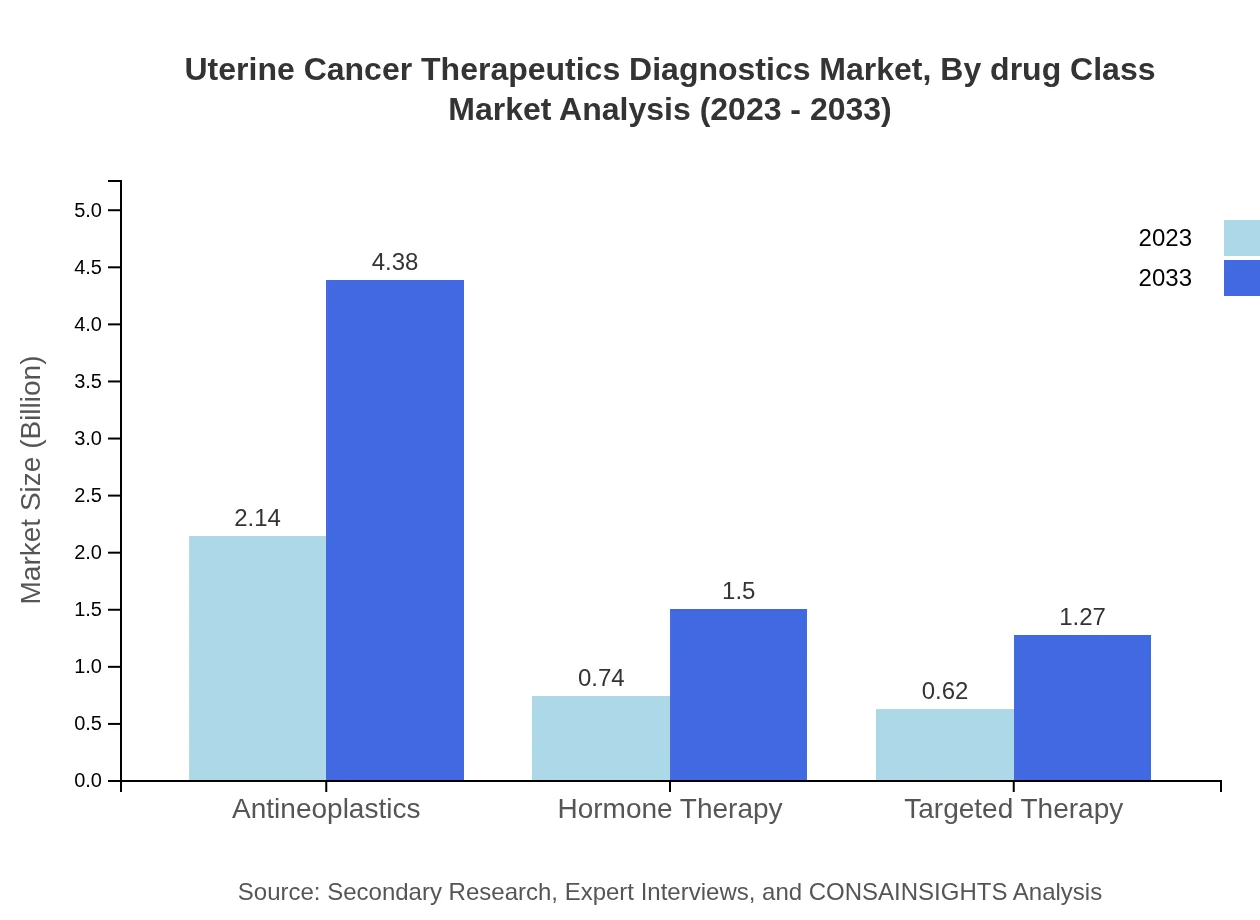
Uterine Cancer Therapeutics Diagnostics Market Analysis By Drug Class
The market is segmented by drug class, with chemotherapy leading both in market size and therapeutic share. The rise of immunotherapies and targeted drugs reflects ongoing innovations that redefine treatment paradigms in uterine cancer diagnostics and therapeutics.
Uterine Cancer Therapeutics Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Uterine Cancer Therapeutics Diagnostics Industry
Roche:
Roche is a leader in the development of diagnostic solutions for cancer treatment and has contributed significantly to uterine cancer therapeutics through innovative research and partnerships.Merck & Co.:
Merck is prominent in the pharmaceutical industry, offering advanced therapeutic products including hormone therapies and immuno-oncology treatments for uterine cancer.Bristol Myers Squibb:
Bristol Myers Squibb is dedicated to transforming treatment through cutting-edge therapies and has made notable strides in immunotherapy for various cancers, including uterine cancer.Novartis:
Novartis focuses on extensive cancer therapeutic research, offering targeted therapies that have shown effectiveness in treating various types of cancers, including uterine cancer.AbbVie:
AbbVie is recognized for its contribution to the field of oncology with a range of therapeutic options designed specifically for cancer treatment, including uterine cancer.We're grateful to work with incredible clients.









FAQs
What is the market size of uterine Cancer Therapeutics Diagnostics?
The uterine cancer therapeutics diagnostics market is valued at approximately $3.5 billion in 2023, with a projected CAGR of 7.2% from 2023 to 2033. This rapid growth indicates a rising demand for advanced diagnostics and therapeutics.
What are the key market players or companies in the uterine Cancer Therapeutics Diagnostics industry?
Key market players in the uterine cancer therapeutics diagnostics industry include major pharmaceutical firms and biotech companies specializing in oncology. These companies play significant roles in developing innovative diagnostic tools and treatment options, driving market advancement.
What are the primary factors driving the growth in the uterine Cancer Therapeutics Diagnostics industry?
Growth is driven by increasing incidence rates of uterine cancer, advancements in diagnostics technology, and rising patient awareness. The trend toward personalized medicine, along with supportive healthcare policies for cancer treatment, further propels market expansion.
Which region is the fastest Growing in the uterine Cancer Therapeutics Diagnostics?
The fastest-growing region is Europe, which is expected to grow from $1.04 billion in 2023 to $2.12 billion by 2033. Asia Pacific is also significant, growing from $0.63 billion to $1.29 billion during the same period.
Does ConsaInsights provide customized market report data for the uterine Cancer Therapeutics Diagnostics industry?
Yes, Consainsights offers customized market reports tailored to specific requirements in the uterine cancer therapeutics diagnostics industry. These reports can provide detailed insights and data tailored to the needs of different stakeholders.
What deliverables can I expect from this uterine Cancer Therapeutics Diagnostics market research project?
Deliverables typically include a comprehensive report with market size, growth projections, segment analyses, and insights on key players. Additional data can cover market trends, regional insights, and strategic recommendations for stakeholders.
What are the market trends of uterine Cancer Therapeutics Diagnostics?
Market trends include an increase in the adoption of innovative diagnostic techniques like imaging and genetic testing. There is also a significant focus on developing targeted therapies and improving patient outcomes through personalized treatment approaches.