Vaccine Adjuvants Market Report
Published Date: 31 January 2026 | Report Code: vaccine-adjuvants
Vaccine Adjuvants Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Vaccine Adjuvants market, covering its size, growth trends, industry dynamics, and regional insights from 2023 to 2033. The findings aim to equip stakeholders with critical data for informed decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
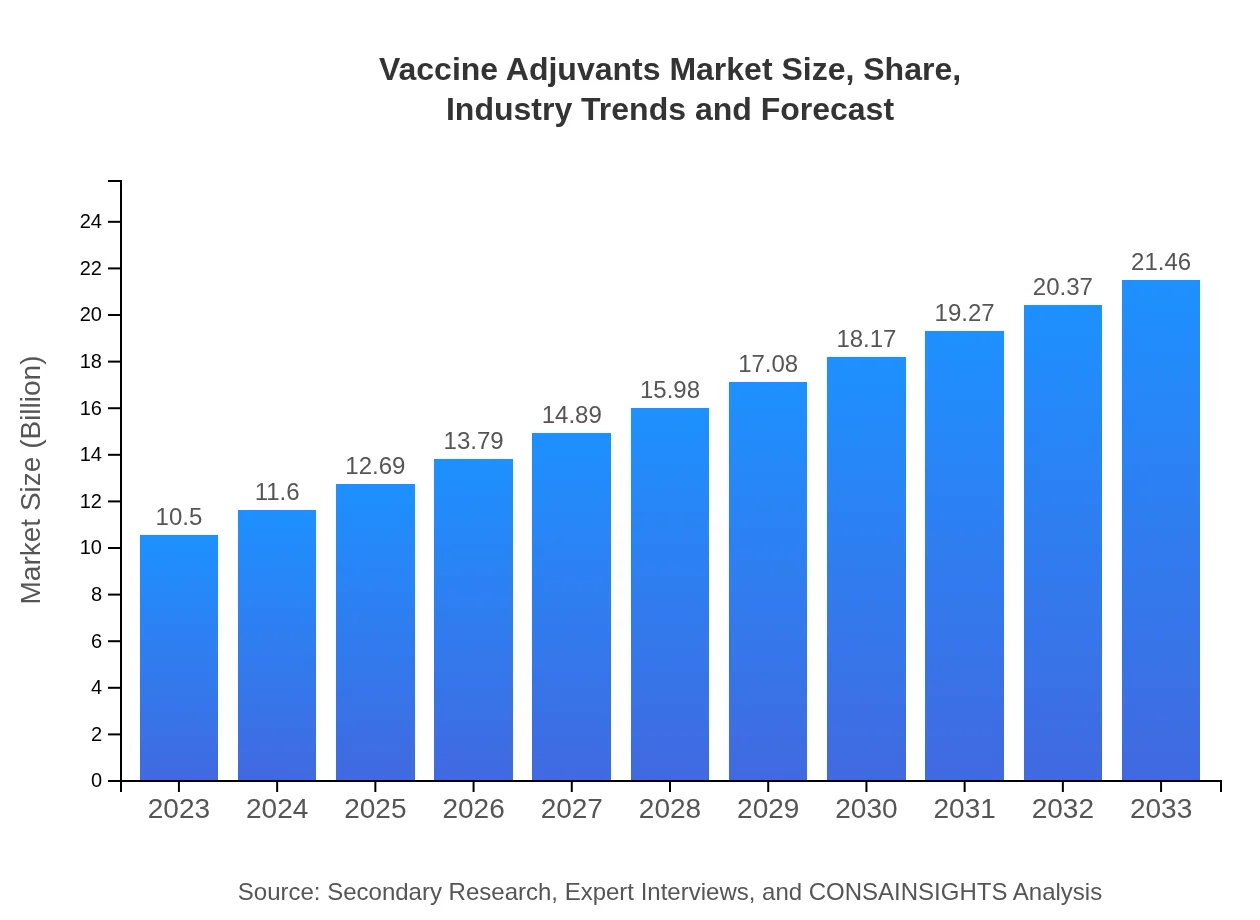
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $21.46 Billion |
| Top Companies | Seqirus, GSK, adjuvants |
| Last Modified Date | 31 January 2026 |
Vaccine Adjuvants Market Overview
Customize Vaccine Adjuvants Market Report market research report
- ✔ Get in-depth analysis of Vaccine Adjuvants market size, growth, and forecasts.
- ✔ Understand Vaccine Adjuvants's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Vaccine Adjuvants
What is the Market Size & CAGR of Vaccine Adjuvants market in 2023?
Vaccine Adjuvants Industry Analysis
Vaccine Adjuvants Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Vaccine Adjuvants Market Analysis Report by Region
Europe Vaccine Adjuvants Market Report:
In Europe, the Vaccine Adjuvants market is valued at $2.91 billion in 2023, with projections to reach $5.95 billion by 2033. The region benefits from a well-established pharmaceutical industry, stringent regulatory frameworks, and a focus on public health initiatives.Asia Pacific Vaccine Adjuvants Market Report:
The Asia-Pacific region shows promising growth, driven by increasing investments in healthcare infrastructure and vaccine development. In 2023, the market is valued at $2.07 billion and is expected to grow to $4.22 billion by 2033. Rising healthcare expenditures and a growing population further enhance the demand for vaccines in this region.North America Vaccine Adjuvants Market Report:
North America remains a significant player with a market size of $3.94 billion in 2023, projected to reach $8.06 billion by 2033. This growth can be attributed to advanced healthcare infrastructure, strong R&D activity, and a robust presence of key market players in the region.South America Vaccine Adjuvants Market Report:
The South American market for Vaccine Adjuvants is valued at $0.70 billion in 2023, with an expected growth to $1.43 billion by 2033. Factors such as improved access to vaccines and governmental initiatives to combat infectious diseases are driving this growth.Middle East & Africa Vaccine Adjuvants Market Report:
The Middle East and Africa market is relatively smaller, with a valuation of $0.89 billion in 2023, expected to grow to $1.81 billion by 2033. Increasing healthcare awareness and initiatives to strengthen vaccination programs are key growth drivers in this region.Tell us your focus area and get a customized research report.
Vaccine Adjuvants Market Analysis By Type
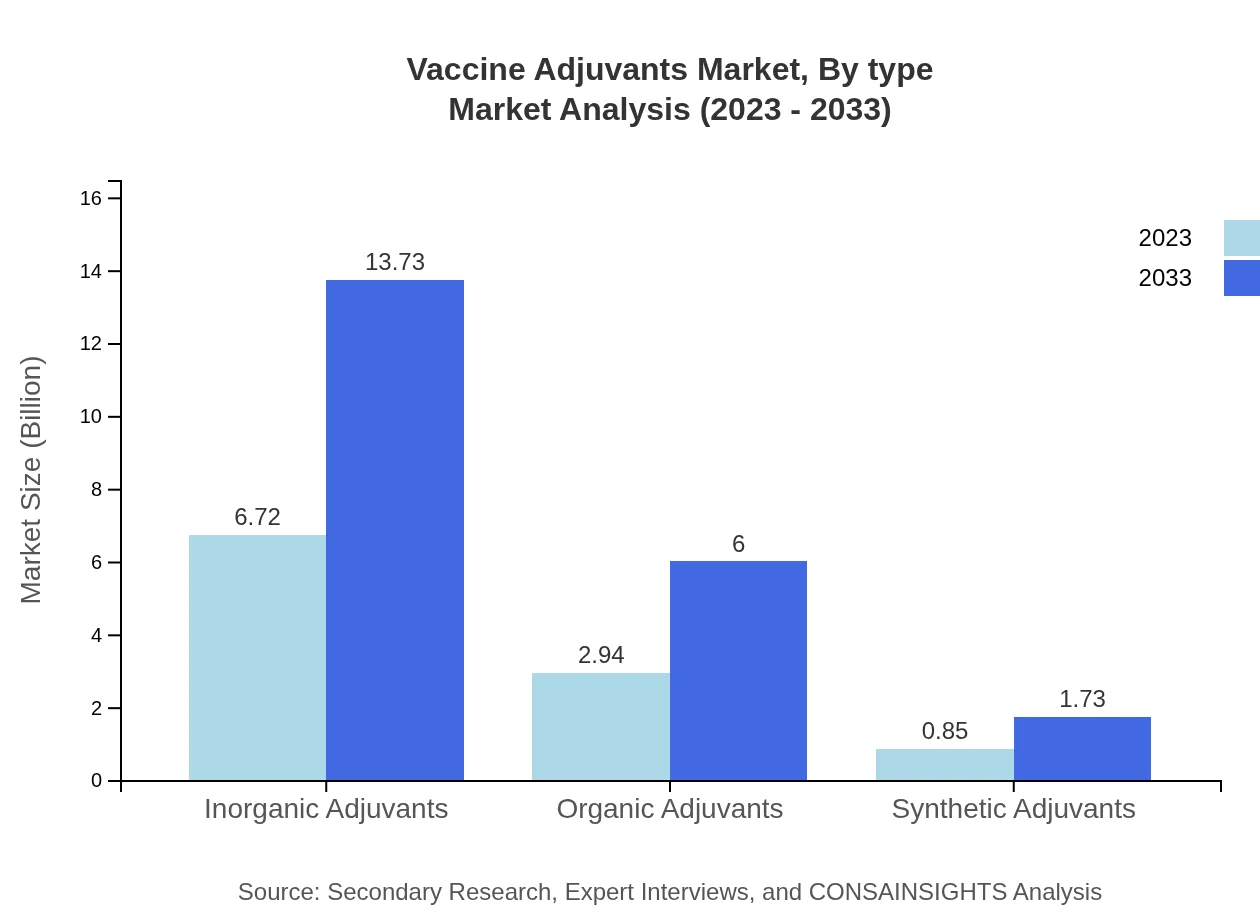
The Vaccine Adjuvants market is predominantly led by inorganic adjuvants, which accounted for 63.96% of the market share in 2023, valued at $6.72 billion, and is projected to be $13.73 billion by 2033. Organic adjuvants follow, representing a 27.97% share, valued at $2.94 billion in 2023 and forecasted to grow to $6.00 billion by 2033. Synthetic adjuvants, while smaller, show significant promise, expected to rise from $0.85 billion in 2023 to $1.73 billion by 2033.
Vaccine Adjuvants Market Analysis By Formulation
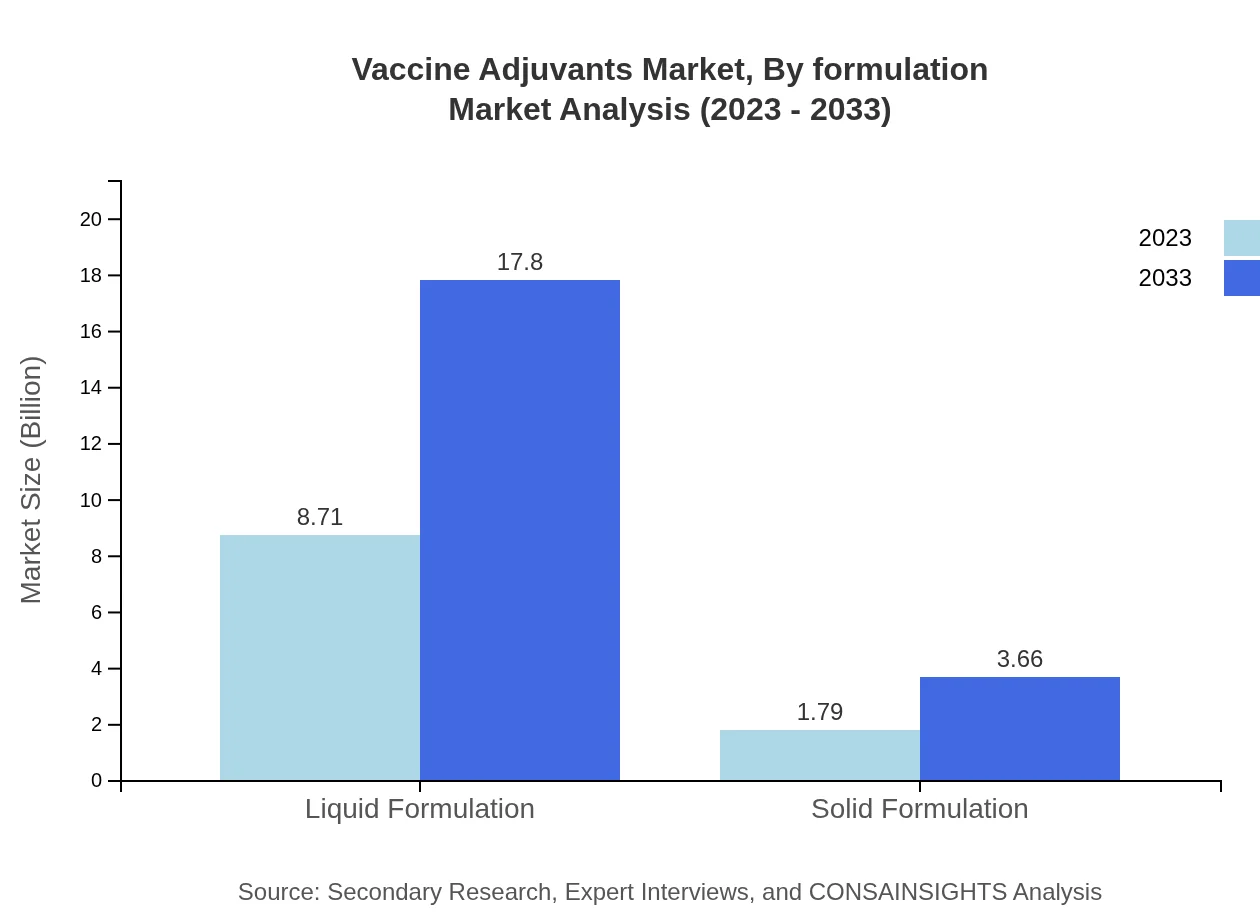
The market segment focuses on liquid and solid formulations, with liquid adjuvants dominating at 82.95% market share, valued at $8.71 billion in 2023, and anticipated to grow to $17.80 billion by 2033. Solid formulations, though lower in share at 17.05%, are expected to reach $3.66 billion in 2033 from $1.79 billion in 2023.
Vaccine Adjuvants Market Analysis By Application
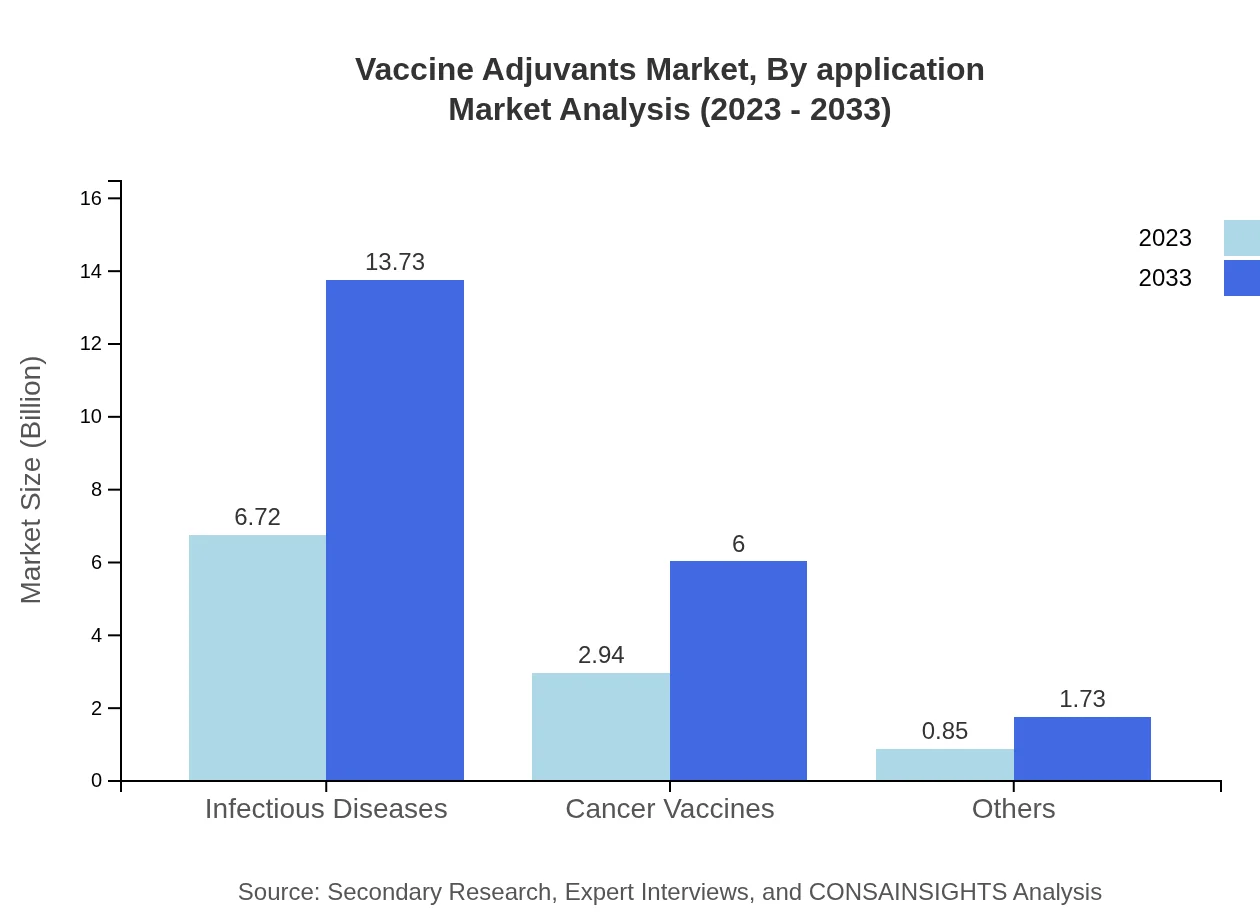
The application-based segmentation highlights the critical roles of adjuvants in infectious diseases and cancer vaccines. Infectious diseases account for 63.96% of the market share at $6.72 billion in 2023, projected to reach $13.73 billion by 2033. Cancer vaccines contribute significantly as well, making up 27.97% with valuations climbing from $2.94 billion to $6.00 billion during the forecast period.
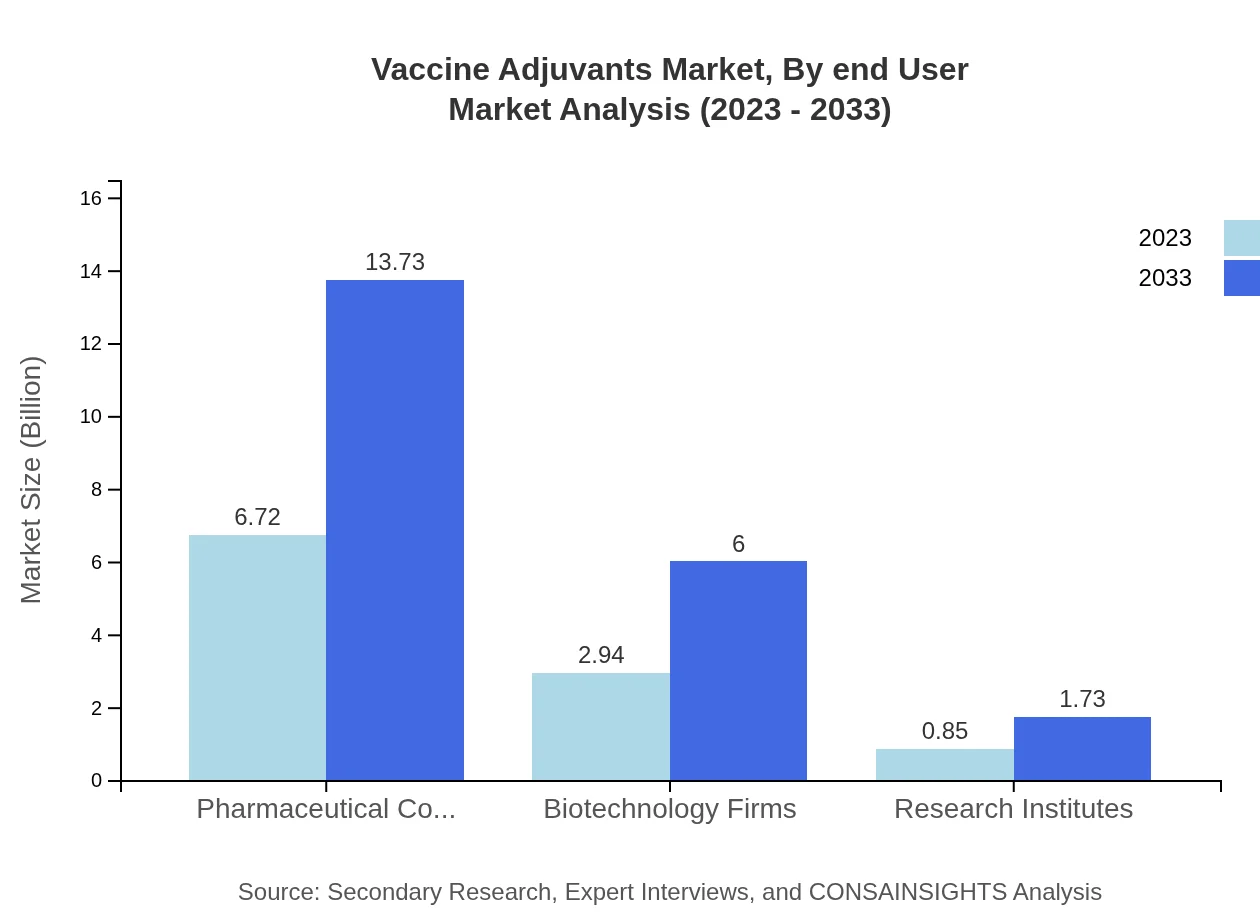
Vaccine Adjuvants Market Analysis By End User
The end-user analysis shows a robust presence of pharmaceutical companies, dominating the segment at 63.96% with a size of $6.72 billion in 2023. Biotechnology firms follow with 27.97% of the market share, valued at $2.94 billion and expected to enhance their share significantly by 2033.
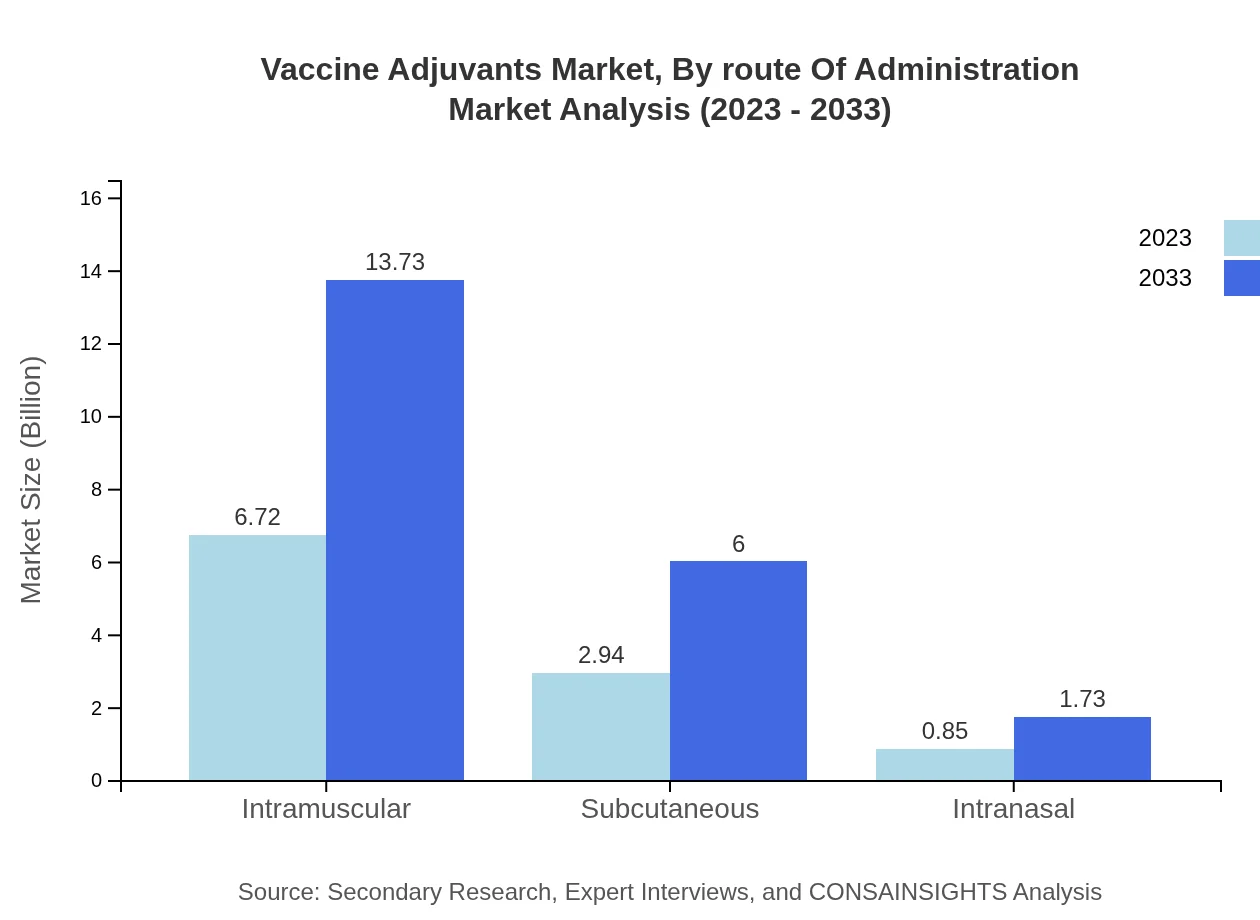
Vaccine Adjuvants Market Analysis By Route Of Administration
Intramuscular administration is the most common route, representing 63.96% of the market size, worth $6.72 billion in 2023 and estimated to increase to $13.73 billion by 2033. Subcutaneous and intranasal routes also play a role, with shares of 27.97% and 8.07%, respectively.
Vaccine Adjuvants Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Vaccine Adjuvants Industry
Seqirus:
A global leader in vaccine innovation, Seqirus excels in the development and production of novel adjuvants that enhance vaccine efficacy.GSK:
GlaxoSmithKline (GSK) is a prominent player in the adjuvants market, known for its extensive research in immunology and innovative adjuvant systems.adjuvants:
A leading player specializing in the design and development of advanced adjuvant technologies for infectious disease and cancer vaccines.We're grateful to work with incredible clients.









FAQs
What is the market size of vaccine Adjuvants?
The global vaccine adjuvants market size is estimated at $10.5 billion in 2023 and is projected to grow at a CAGR of 7.2% through 2033. This growth reflects increasing demand for effective vaccine formulations and advancements in immunological research.
What are the key market players or companies in this vaccine Adjuvants industry?
Key players in the vaccine adjuvants industry include major pharmaceutical companies such as GlaxoSmithKline, Merck, and Novartis, along with biotechnology firms such as Agenus Inc. and Immunotherapies Inc., which contribute to innovative adjuvant products.
What are the primary factors driving the growth in the vaccine Adjuvants industry?
Primary factors driving growth include rising incidence of infectious diseases, innovations in vaccine technologies, increased investments in R&D, and the growing emphasis on preventive healthcare, which has heightened the demand for effective vaccines.
Which region is the fastest Growing in the vaccine Adjuvants?
North America is the fastest-growing region, with market size projected to rise from $3.94 billion in 2023 to $8.06 billion by 2033, driven by advanced healthcare infrastructure and increased vaccination programs.
Does ConsaInsights provide customized market report data for the vaccine Adjuvants industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the vaccine-adjuvants industry, providing insights into niche markets, regional analyses, and emerging trends for more informed decision-making.
What deliverables can I expect from this vaccine Adjuvants market research project?
Expect comprehensive deliverables including detailed market analysis, competitive landscape reports, forecasting models, and actionable insights, enabling stakeholders to understand trends, opportunities, and challenges in the vaccine-adjuvants market.
What are the market trends of vaccine Adjuvants?
Key trends in the vaccine adjuvants market include increased utilization of adjuvants in cancer vaccine development, growth of organic adjuvants, and rising demand for liquid formulations, shaping future vaccine strategies and developments.