Varicella Vaccine Market Report
Published Date: 31 January 2026 | Report Code: varicella-vaccine
Varicella Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report offers a comprehensive analysis of the Varicella Vaccine market, covering the market dynamics, size, growth drivers, and trends from 2023 to 2033. It provides valuable insights into market segmentation, regional performance, and key industry players, along with forecast data to support strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
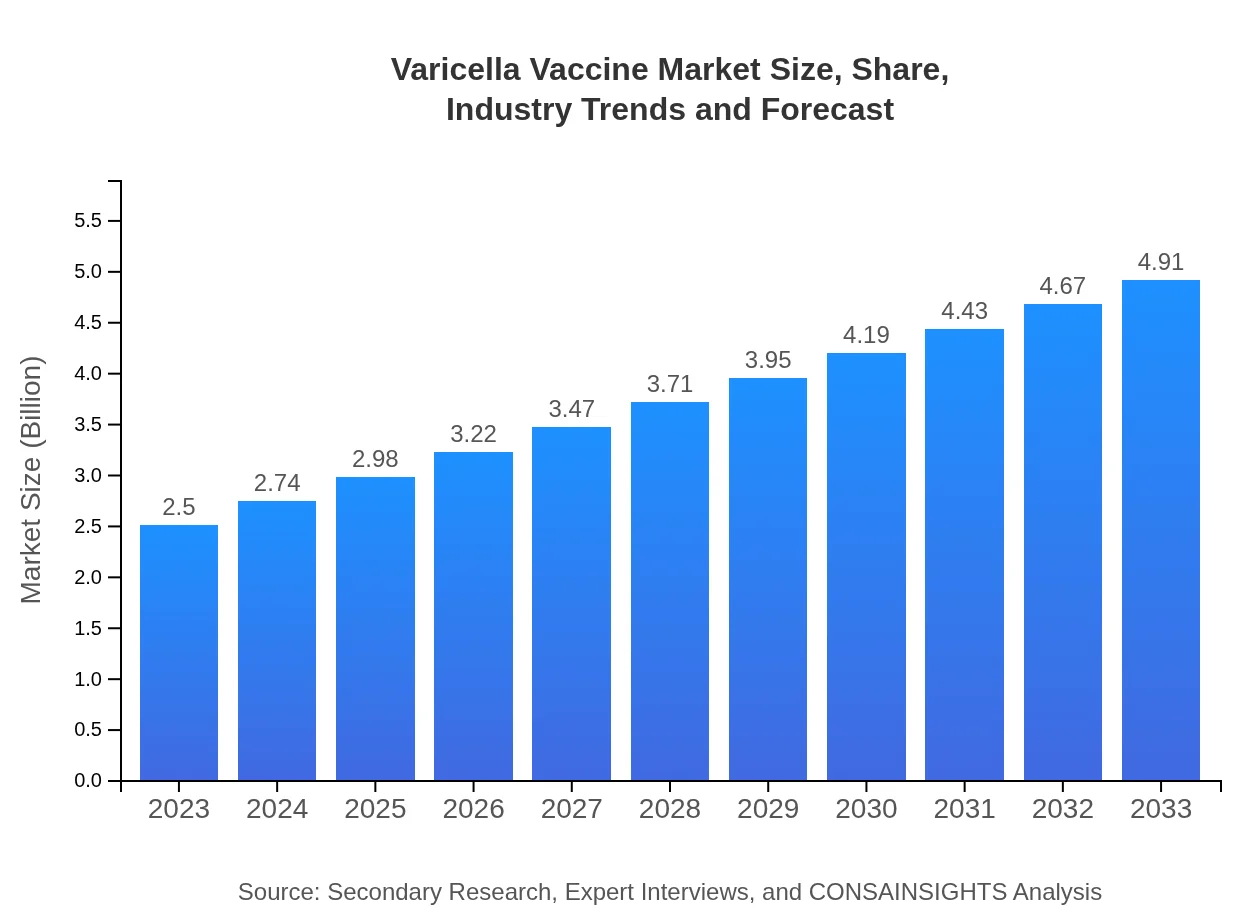
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Merck & Co., Sanofi Pasteur, Pfizer Inc., GlaxoSmithKline plc, AstraZeneca |
| Last Modified Date | 31 January 2026 |
Varicella Vaccine Market Overview
Customize Varicella Vaccine Market Report market research report
- ✔ Get in-depth analysis of Varicella Vaccine market size, growth, and forecasts.
- ✔ Understand Varicella Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Varicella Vaccine
What is the Market Size & CAGR of Varicella Vaccine market in 2023?
Varicella Vaccine Industry Analysis
Varicella Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Varicella Vaccine Market Analysis Report by Region
Europe Varicella Vaccine Market Report:
The European Varicella Vaccine market is forecasted to grow from $0.80 billion in 2023 to $1.57 billion by 2033. Enhanced public health policies and vaccination schedules that include Varicella Vaccines drive this growth, alongside a well-informed populace regarding vaccination benefits.Asia Pacific Varicella Vaccine Market Report:
In the Asia Pacific region, the Varicella Vaccine market is expected to grow from $0.44 billion in 2023 to $0.86 billion by 2033. Factors driving growth include rising vaccination awareness and government initiatives promoting immunization. The growing healthcare infrastructure in countries like India and China also strengthens market prospects.North America Varicella Vaccine Market Report:
North America holds a significant portion of the Varicella Vaccine market, expected to grow from $0.93 billion in 2023 to $1.82 billion by 2033. The region benefits from advanced healthcare systems, high vaccination rates, and continued public health initiatives aimed at disease prevention.South America Varicella Vaccine Market Report:
The South American market for Varicella Vaccines is projected to expand from $0.14 billion in 2023 to $0.28 billion by 2033. This growth is attributed to increased investment in healthcare and vaccination programs by governments, supported by international health organizations.Middle East & Africa Varicella Vaccine Market Report:
The Middle East and Africa region's market is anticipated to grow from $0.19 billion in 2023 to $0.38 billion by 2033. Rising healthcare accessibility and education about the importance of vaccinations are crucial for this growth, despite challenges related to infrastructure in some areas.Tell us your focus area and get a customized research report.
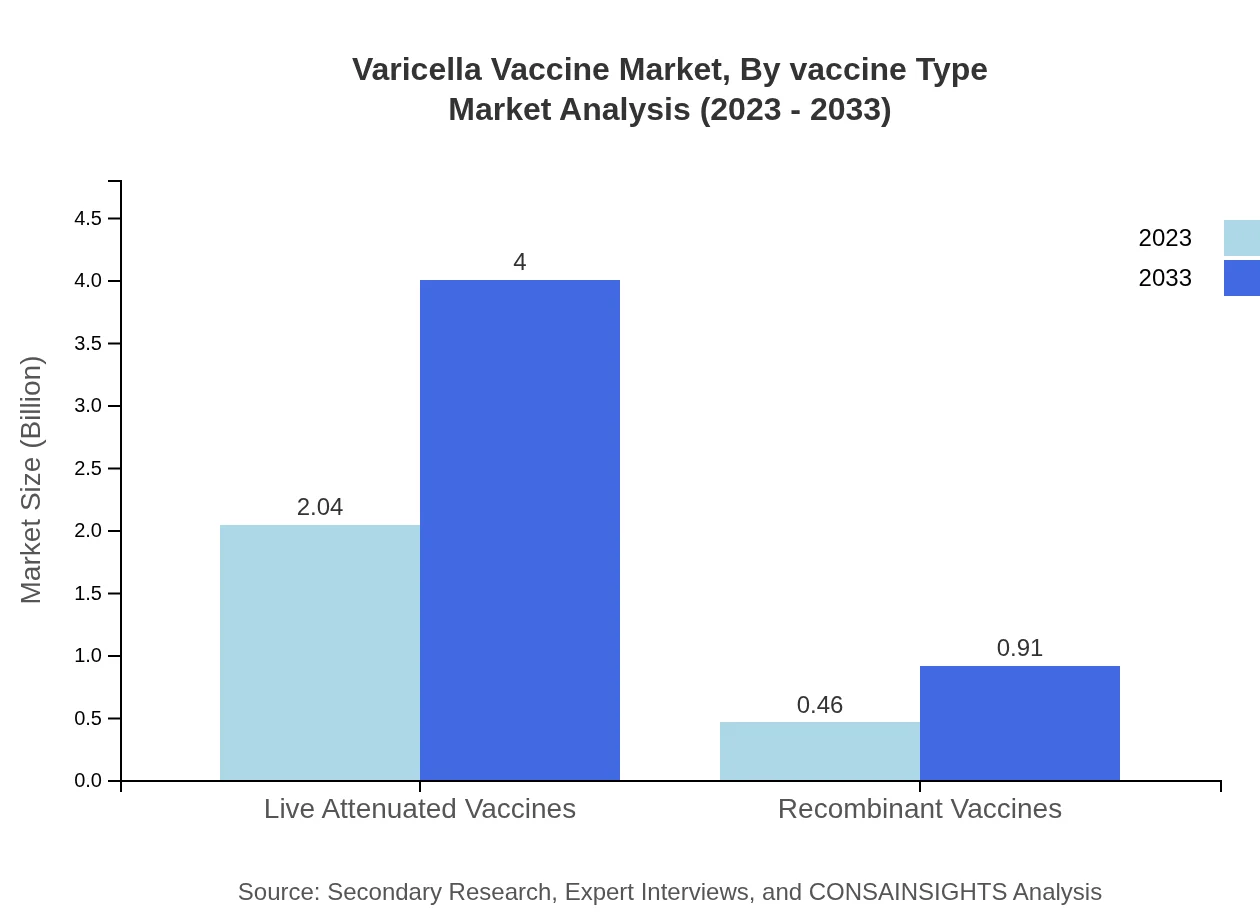
Varicella Vaccine Market Analysis By Vaccine Type
The Varicella Vaccine market by vaccine type showcases significant growth for live attenuated vaccines, projected to increase from $2.04 billion in 2023 to $4.00 billion by 2033, holding 81.41% market share throughout this period. Recombinant vaccines, though smaller in share, are also gaining traction, with a market size expected to rise from $0.46 billion in 2023 to $0.91 billion by 2033, representing 18.59% share.
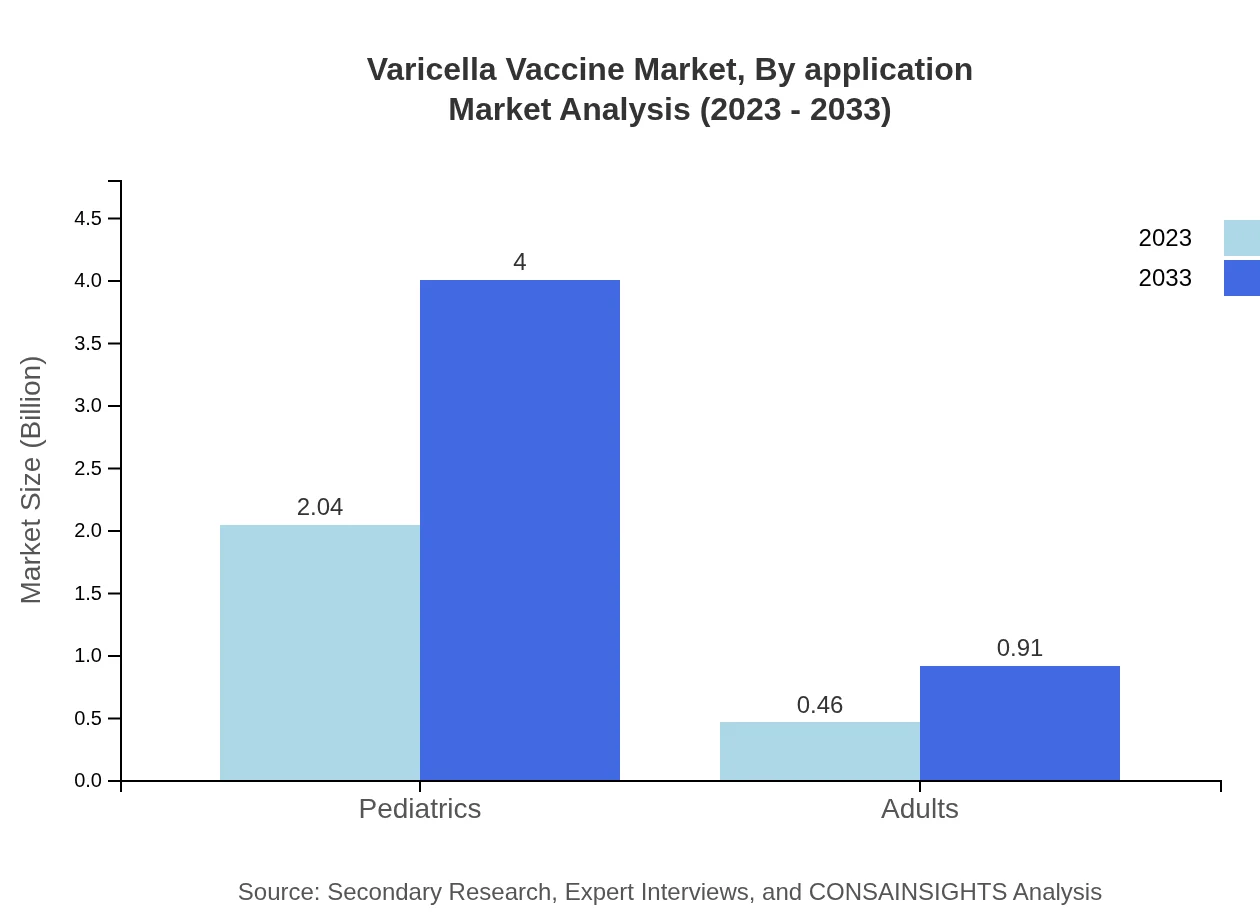
Varicella Vaccine Market Analysis By Application
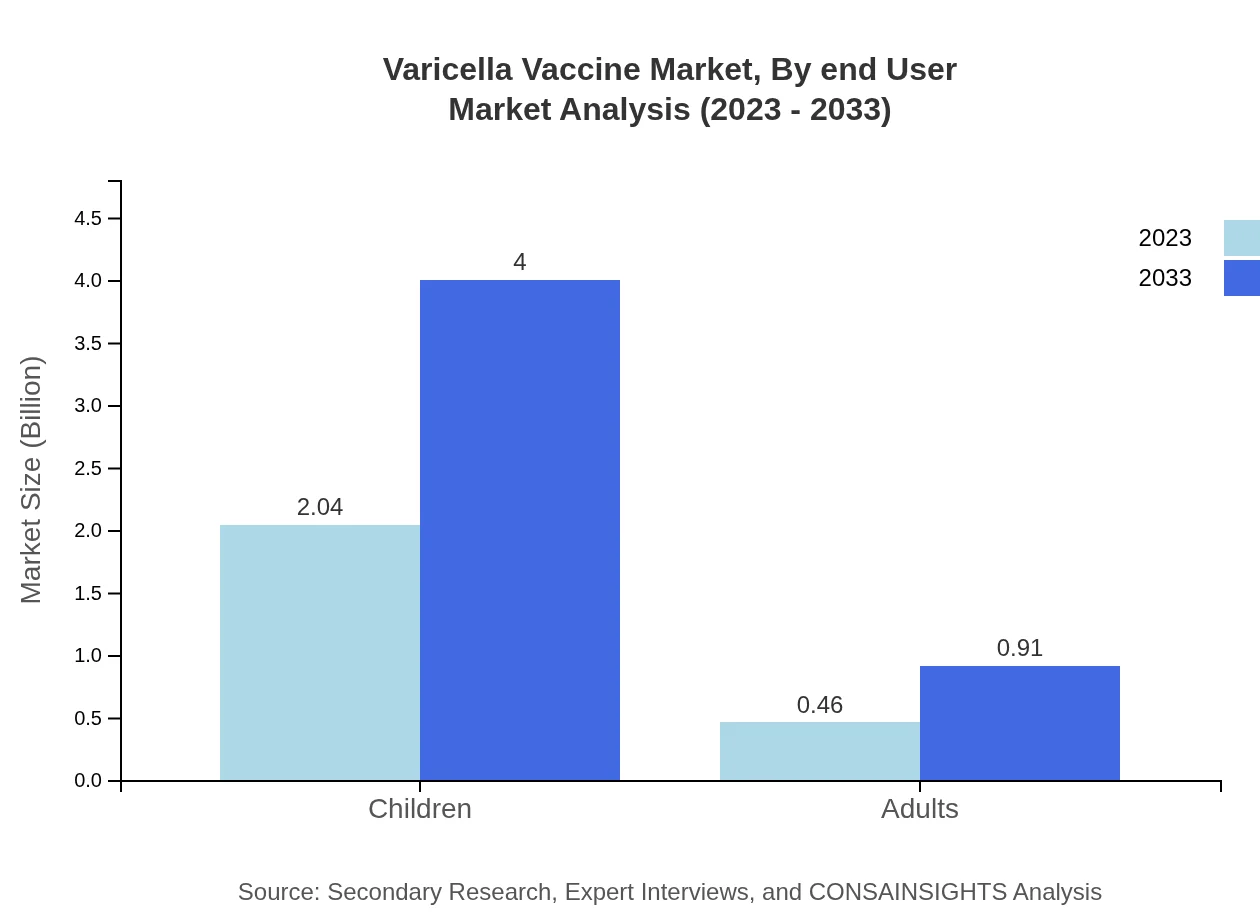
The pediatric segment leads the Varicella Vaccine market, with a substantial size of $2.04 billion in 2023, expected to grow to $4.00 billion by 2033, maintaining an 81.41% share of the market. The adult segment is also showing growth potential, increasing from $0.46 billion to $0.91 billion and holding an 18.59% share.
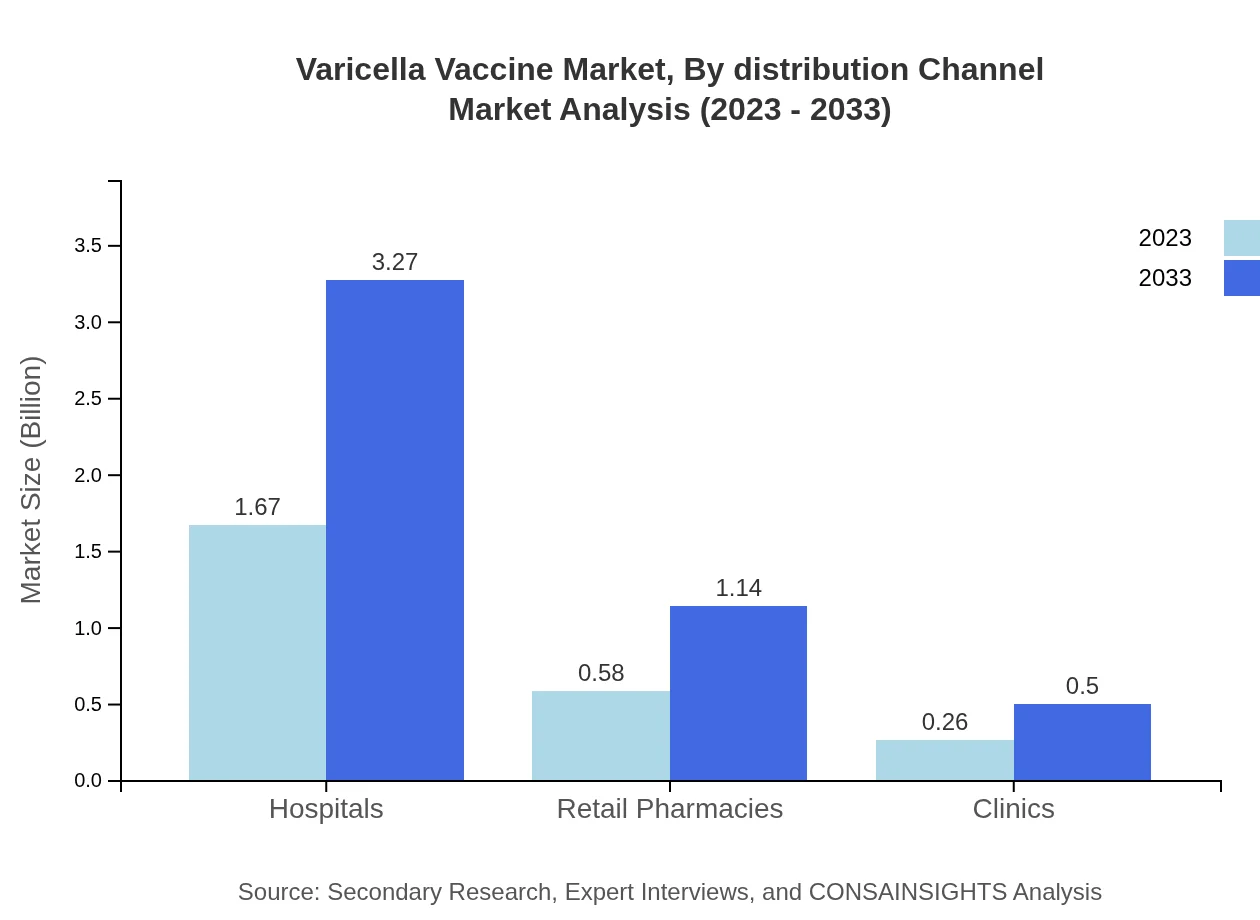
Varicella Vaccine Market Analysis By Distribution Channel
In terms of distribution channels, hospitals command the most significant market share, with a size of $1.67 billion in 2023, projected to reach $3.27 billion by 2033 (66.65% share). Retail pharmacies and clinics are also essential, with expected growth trajectories from $0.58 billion to $1.14 billion and $0.26 billion to $0.50 billion, accounting for 23.14% and 10.21% shares, respectively.
Varicella Vaccine Market Analysis By End User
The end-user analysis indicates healthcare providers are the primary users of the Varicella Vaccine, reflecting the growing trend towards preventive healthcare. The market is expanding as healthcare facilities prioritize vaccinations as part of routine healthcare services, thus ensuring improved health outcomes for the target populations.
Varicella Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Varicella Vaccine Industry
Merck & Co.:
A leading player known for the production of the Varivax vaccine, Merck focuses on advancing vaccine technologies and expanding access to vaccination programs across various demographics.Sanofi Pasteur:
A major player in the vaccine segment, Sanofi Pasteur offers comprehensive immunization solutions and emphasizes research and development for enhancing vaccine efficacy.Pfizer Inc.:
Pfizer is renowned for its innovative approach in healthcare, contributing to vaccine development and distribution, aimed at create a healthier future through effective immunization strategies.GlaxoSmithKline plc:
GSK engages in developing vaccines with a strong portfolio in pediatric vaccines, ensuring safety and efficacy to cater to immunization needs globally.AstraZeneca:
Known for its significant contributions to global health, AstraZeneca is expanding its vaccine offerings and improving healthcare access through strategic partnerships.We're grateful to work with incredible clients.









FAQs
What is the market size of varicella Vaccine?
The global varicella vaccine market is valued at approximately $2.5 billion in 2023, with a projected CAGR of 6.8% by 2033. This indicates strong growth driven by vaccination initiatives and increased awareness of varicella-related health risks.
What are the key market players or companies in this varicella Vaccine industry?
Key players in the varicella vaccine market include pharmaceutical giants like Merck & Co., GlaxoSmithKline, and Sanofi. These companies lead in vaccine development, distribution, and marketing, ensuring global accessibility to varicella vaccination.
What are the primary factors driving the growth in the varicella Vaccine industry?
The growth of the varicella vaccine market is primarily driven by increasing vaccination rates, rising public awareness about the health impacts of varicella, and initiatives from health organizations promoting immunization against contagious diseases like chickenpox.
Which region is the fastest Growing in the varicella Vaccine?
The fastest-growing region in the varicella vaccine market is projected to be Europe, increasing from $0.80 billion in 2023 to $1.57 billion by 2033. Other notable regions include North America and Asia Pacific showing significant growth.
Does ConsaInsights provide customized market report data for the varicella Vaccine industry?
Yes, ConsaInsights offers customized market report data for the varicella vaccine industry. Clients can request tailored insights based on specific needs or focus areas for a comprehensive understanding of market dynamics.
What deliverables can I expect from this varicella Vaccine market research project?
Deliverables from the varicella vaccine market research project include detailed market analysis reports, trend overviews, forecasts for market size, regional insights, and profiles of key market players, ensuring thorough market intelligence.
What are the market trends of varicella Vaccine?
Current trends in the varicella vaccine market include increased adoption of live attenuated vaccines, expansion in pediatric vaccination programs, a shift towards recombinant vaccines, and advancements in healthcare delivery through retail pharmacies and clinics.