Ventricular Assist Device Market Report
Published Date: 31 January 2026 | Report Code: ventricular-assist-device
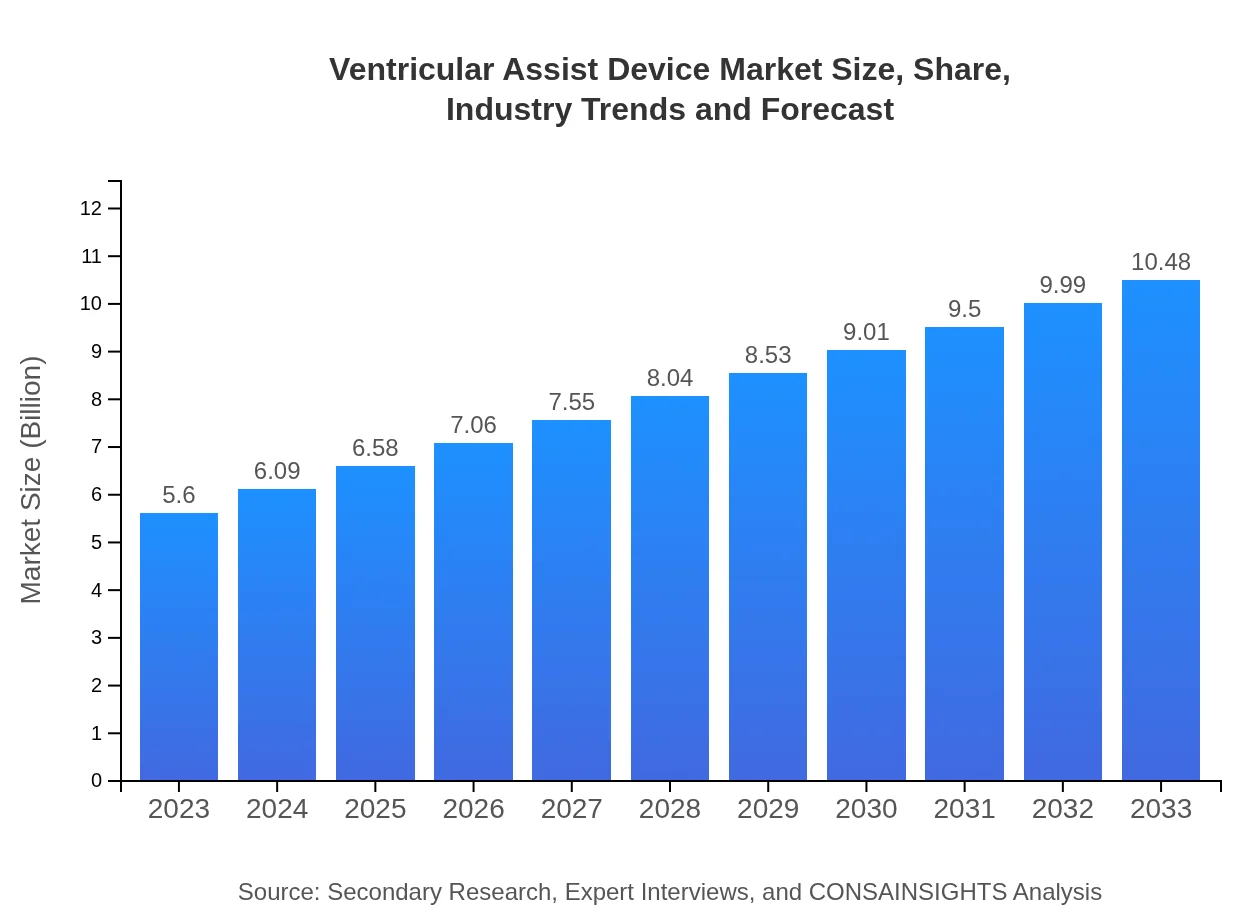
Ventricular Assist Device Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Ventricular Assist Device (VAD) market, including market size forecasts, key trends, and insights from 2023 to 2033, with a focus on regional and segment-specific growth opportunities.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.3% |
| 2033 Market Size | $10.48 Billion |
| Top Companies | Abbott Laboratories, Medtronic , Boston Scientific, Cordis |
| Last Modified Date | 31 January 2026 |
Ventricular Assist Device Market Overview
Customize Ventricular Assist Device Market Report market research report
- ✔ Get in-depth analysis of Ventricular Assist Device market size, growth, and forecasts.
- ✔ Understand Ventricular Assist Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Ventricular Assist Device
What is the Market Size & CAGR of Ventricular Assist Device market in 2033?
Ventricular Assist Device Industry Analysis
Ventricular Assist Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Ventricular Assist Device Market Analysis Report by Region
Europe Ventricular Assist Device Market Report:
The European market for Ventricular Assist Devices is projected to expand from $1.44 billion in 2023 to $2.69 billion by 2033. Major factors driving this growth include a robust healthcare framework, increasing approvals for new devices, and ongoing clinical research. Countries like Germany, France, and the UK are leading in technological adoption and patient outcomes.Asia Pacific Ventricular Assist Device Market Report:
In the Asia Pacific region, the Ventricular Assist Device market is projected to grow from $1.16 billion in 2023 to $2.16 billion by 2033, driven by increasing healthcare investments and the rising prevalence of cardiovascular diseases. The introduction of innovative VAD technologies is expected to boost patient adoption rates, particularly in emerging markets such as India and China where healthcare access is improving.North America Ventricular Assist Device Market Report:
North America is characterized as the largest market for Ventricular Assist Devices, evaluated at $2.03 billion in 2023 and projected to reach $3.81 billion by 2033. The growth is primarily attributed to advanced healthcare systems, significant R&D funding, and the adoption of innovative technologies. The increasing geriatric population and related heart conditions further enhance market potential.South America Ventricular Assist Device Market Report:
South America presents a steady growth forecast for the Ventricular Assist Device market, anticipated to expand from $0.28 billion in 2023 to $0.52 billion by 2033. Factors contributing to this growth include the rising awareness of heart diseases and improved healthcare infrastructure, particularly in Brazil and Argentina, although economic fluctuations may pose challenges.Middle East & Africa Ventricular Assist Device Market Report:
The Middle East and Africa region is seeing a gradual increase in market development, with a growth from $0.69 billion in 2023 to $1.30 billion by 2033. The growth in this region is primarily fueled by improvements in healthcare access and increasing investments in medical technologies, despite existing challenges such as limited resources and high costs.Tell us your focus area and get a customized research report.
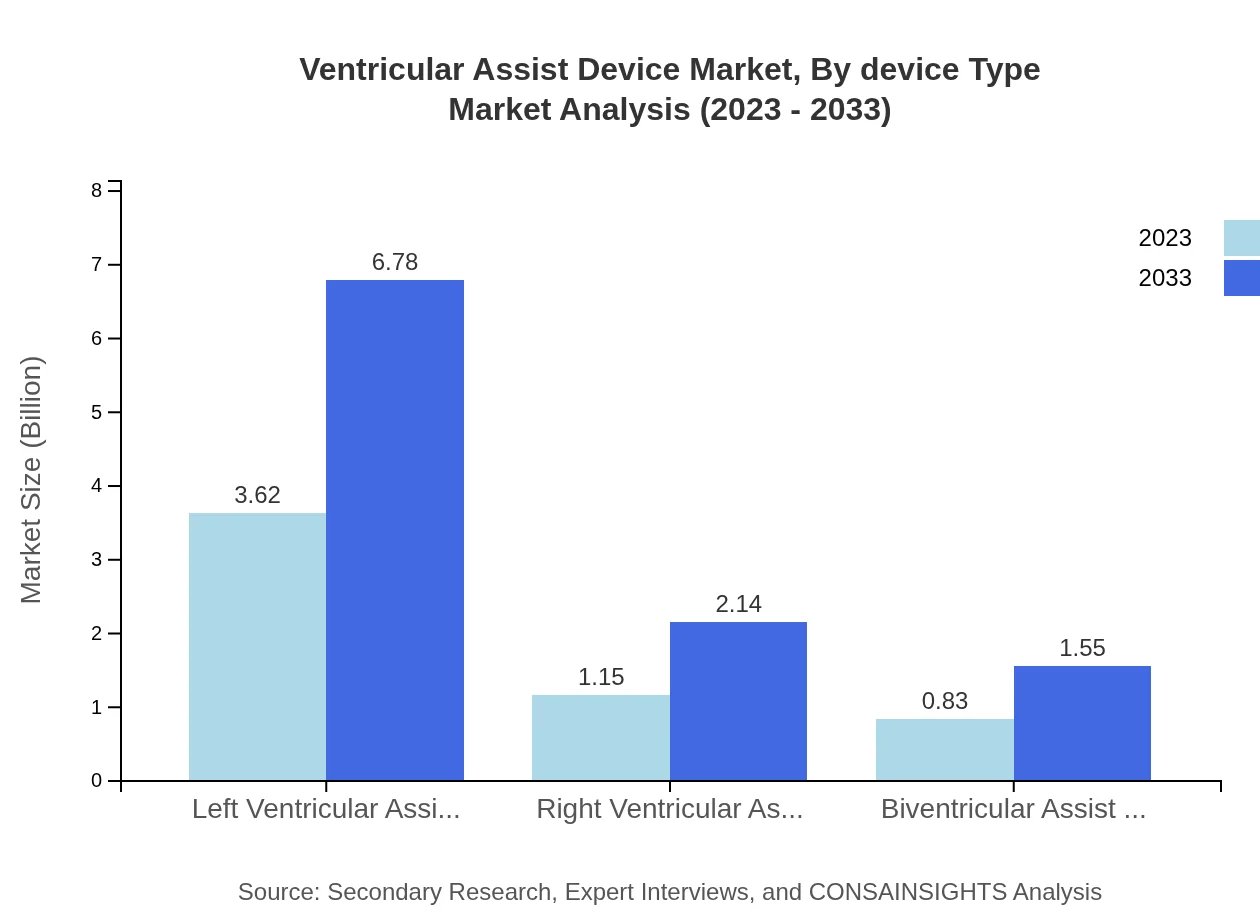
Ventricular Assist Device Market Analysis By Device Type
The market is dominated by Left Ventricular Assist Devices (LVAD), which accounted for $3.62 billion in 2023 and is expected to grow to $6.78 billion by 2033. Right Ventricular Assist Devices (RVAD) and Biventricular Assist Devices (BiVAD) follow, reflecting growing trends in dual ventricle support as their respective markets grow steadily from $1.15 billion to $2.14 billion (RVAD) and $0.83 billion to $1.55 billion (BiVAD) over the same period.
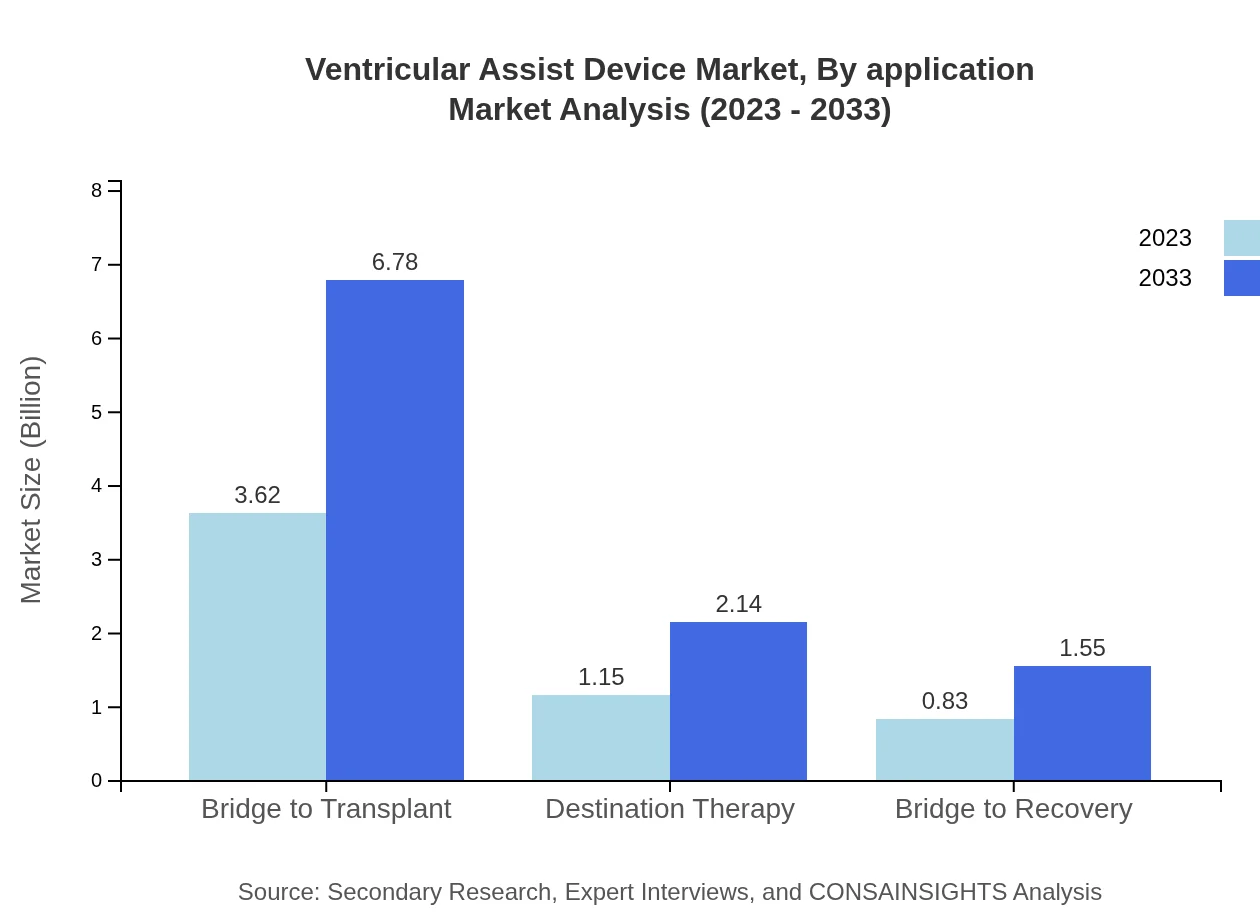
Ventricular Assist Device Market Analysis By Application
Applications for Ventricular Assist Devices include Bridge to Transplant, Destination Therapy, and Bridge to Recovery. The Bridge to Transplant segment currently leads the market at $3.62 billion in 2023, projected to grow to $6.78 billion by 2033, maintaining a significant share. Destination Therapy accounts for $1.15 billion with a growth forecast to $2.14 billion, whereas Bridge to Recovery is anticipated to grow from $0.83 billion to $1.55 billion.
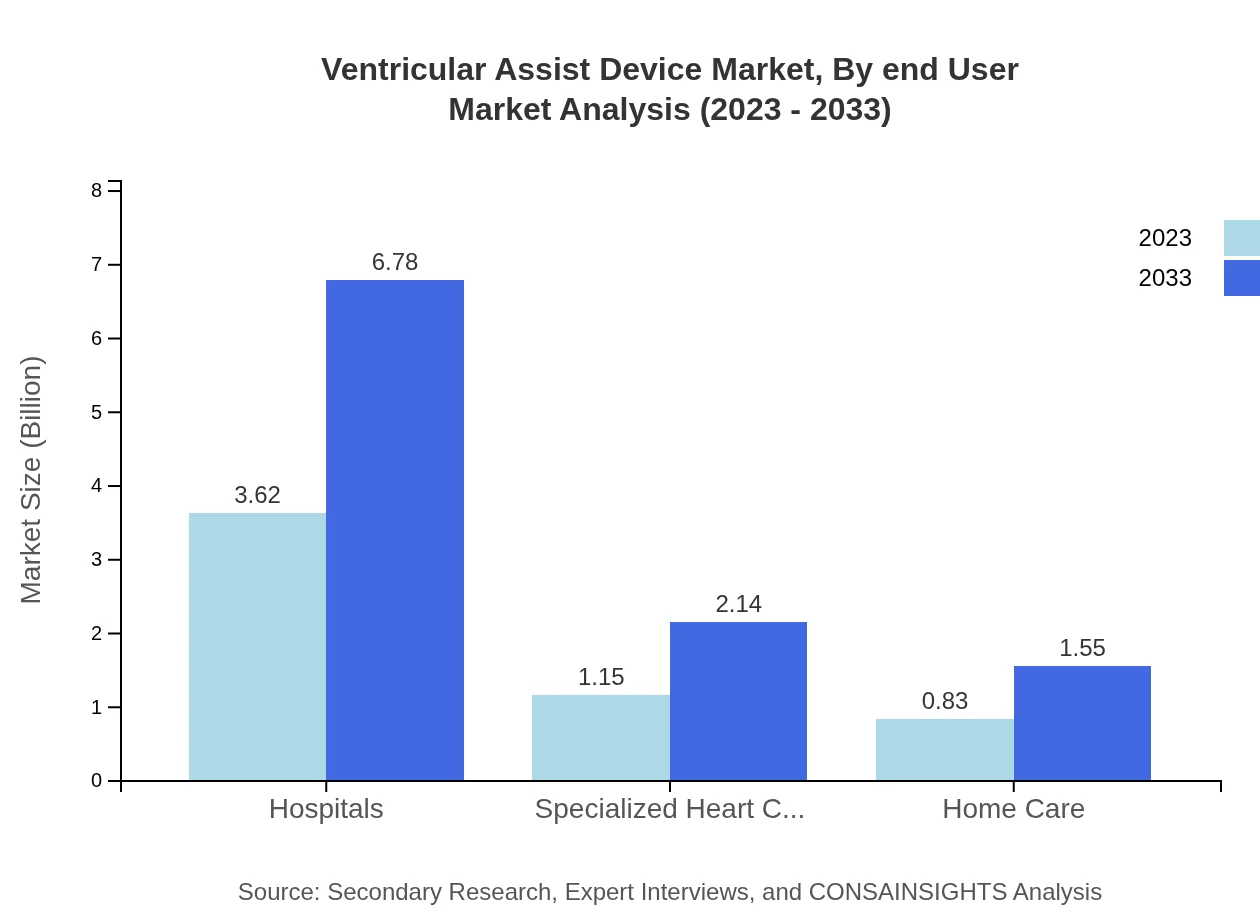
Ventricular Assist Device Market Analysis By End User
The end-user segment highlights Hospitals as the primary users of VADs, with a market size of $3.62 billion in 2023, forecasted to reach $6.78 billion by 2033. Specialized Heart Centers also represent significant growth prospects with their market anticipated to double from $1.15 billion to $2.14 billion over the same period. Home Care, while smaller, signifies a growing demand with forecasts from $0.83 billion to $1.55 billion.
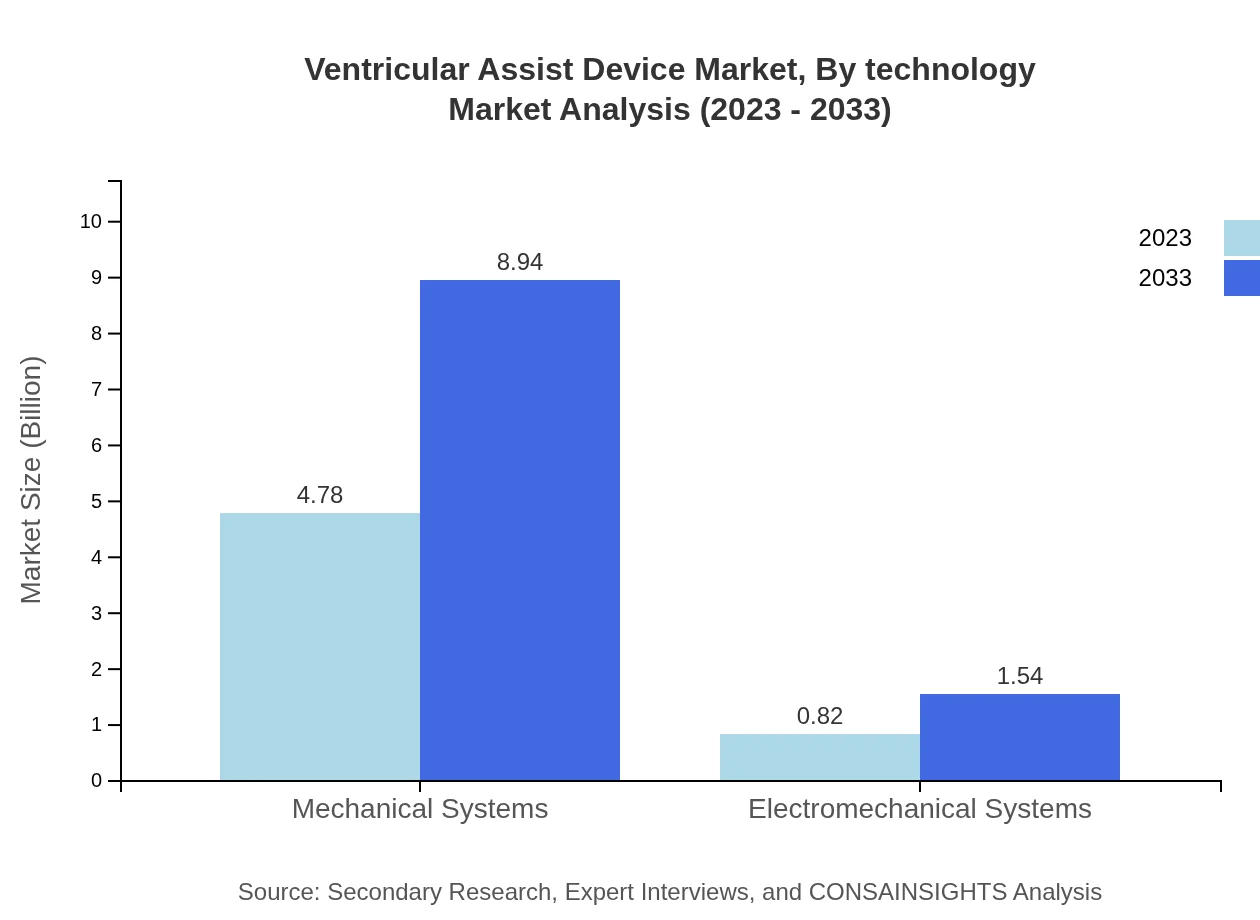
Ventricular Assist Device Market Analysis By Technology
The technological breakdown of the VAD market categorizes products into Mechanical and Electromechanical systems. Mechanical Systems comfortably dominate the segment, accounting for $4.78 billion in size by 2023 and projected to grow to $8.94 billion. In contrast, Electromechanical Systems represent a smaller share, with an increase from $0.82 billion to $1.54 billion expected over the forecast period.
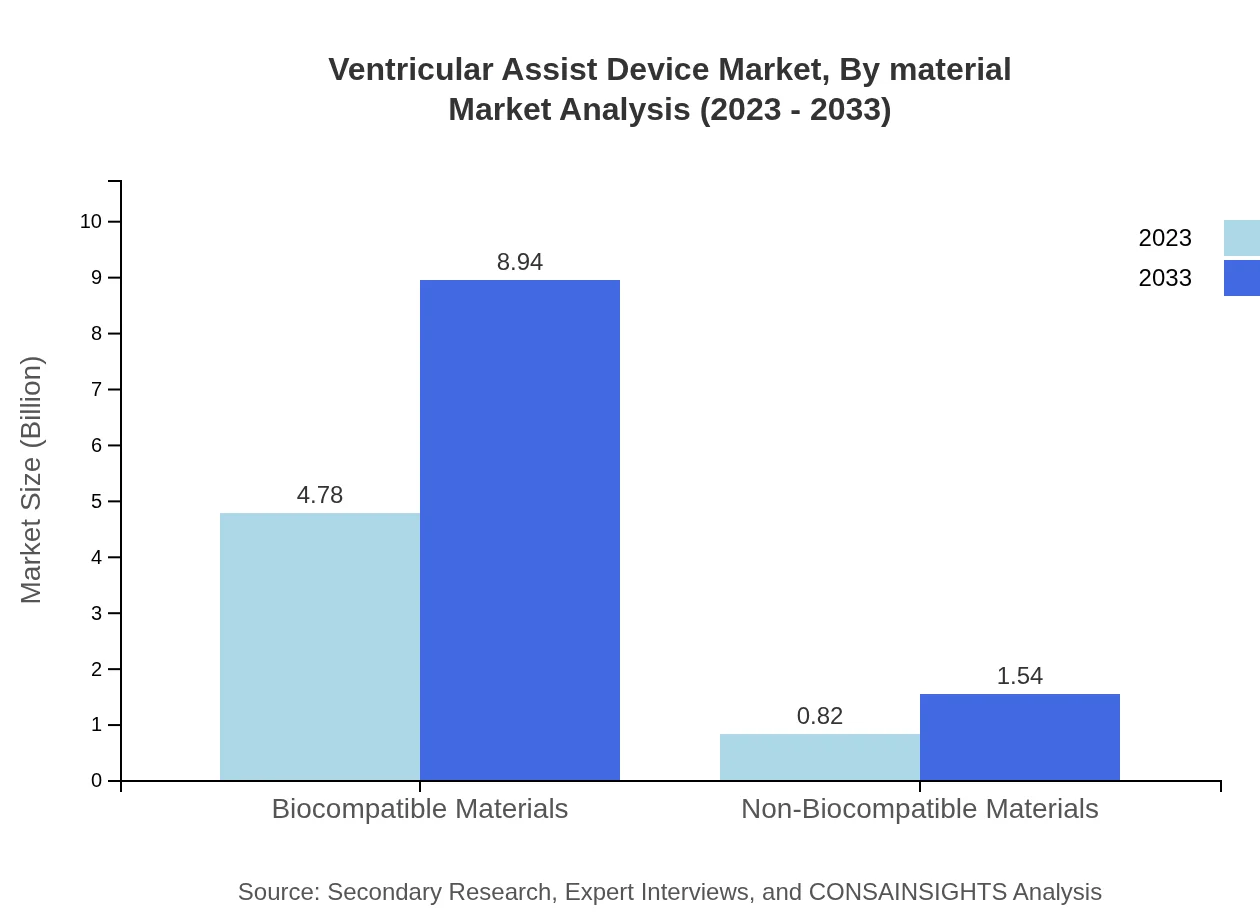
Ventricular Assist Device Market Analysis By Material
Market analysis by material indicates Biocompatible Materials leading the segment, valued at $4.78 billion in 2023 and expected to grow to $8.94 billion by 2033. Non-Biocompatible Materials are expected to increase from $0.82 billion to $1.54 billion, albeit representing a smaller portion of the overall market.
Ventricular Assist Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Ventricular Assist Device Industry
Abbott Laboratories:
Abbott is a global healthcare leader focused on innovative devices for chronic conditions, prominent in the VAD market with its HeartMate devices which are widely recognized and utilized.Medtronic :
Medtronic is a diversified healthcare company that provides innovative medical technologies and services, leading in the VAD arena with advanced device solutions for heart failure management.Boston Scientific:
Boston Scientific is dedicated to transforming lives through innovative medical solutions. The company has made significant strides within the VAD market through its advanced cardiac technologies.Cordis:
Cordis specializes in innovative cardiology and vascular solutions, contributing to the VAD market with a focus on improving the standards of care through quality products.We're grateful to work with incredible clients.









FAQs
What is the market size of ventricular Assist Device?
The global ventricular assist device market is estimated to be valued at approximately $5.6 billion in 2023, with a projected compound annual growth rate (CAGR) of 6.3% leading up to 2033.
What are the key market players or companies in this ventricular Assist Device industry?
Key players in the ventricular assist device market include major medical technology companies focused on cardiovascular innovations. Establishing a portfolio in this domain highlights their commitment to improving heart failure treatment options.
What are the primary factors driving the growth in the ventricular Assist Device industry?
Growth factors in the ventricular assist device industry include technological advancements, rising prevalence of heart diseases, and increasing acceptance of implantable devices for long-term heart management.
Which region is the fastest Growing in the ventricular Assist Device market?
The Asia Pacific region is the fastest-growing in the ventricular assist device market, anticipated to expand from $1.16 billion in 2023 to $2.16 billion by 2033, reflecting a significant growth trajectory.
Does ConsaInsights provide customized market report data for the ventricular Assist Device industry?
Yes, ConsaInsights offers customized market report data tailored to client needs in the ventricular assist device sector, providing comprehensive insights based on specific research parameters.
What deliverables can I expect from this ventricular Assist Device market research project?
Deliverables from the ventricular assist device market research project include detailed market analysis, growth projections, competitive landscape insights, and regional breakdowns presented in a user-friendly report format.
What are the market trends of ventricular Assist Device?
Key market trends include increasing adoption of miniaturized devices, innovations in biocompatible materials, and a shift towards outpatient management using home care solutions, enhancing patient care and experience.