Viral Clearance Market Report
Published Date: 31 January 2026 | Report Code: viral-clearance
Viral Clearance Market Size, Share, Industry Trends and Forecast to 2033
This report comprehensively analyzes the Viral Clearance market, focusing on industry insights, market size, segmentation, regional dynamics, and competitive landscape. It provides forecasts from 2023 to 2033, assessing growth opportunities and challenges.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
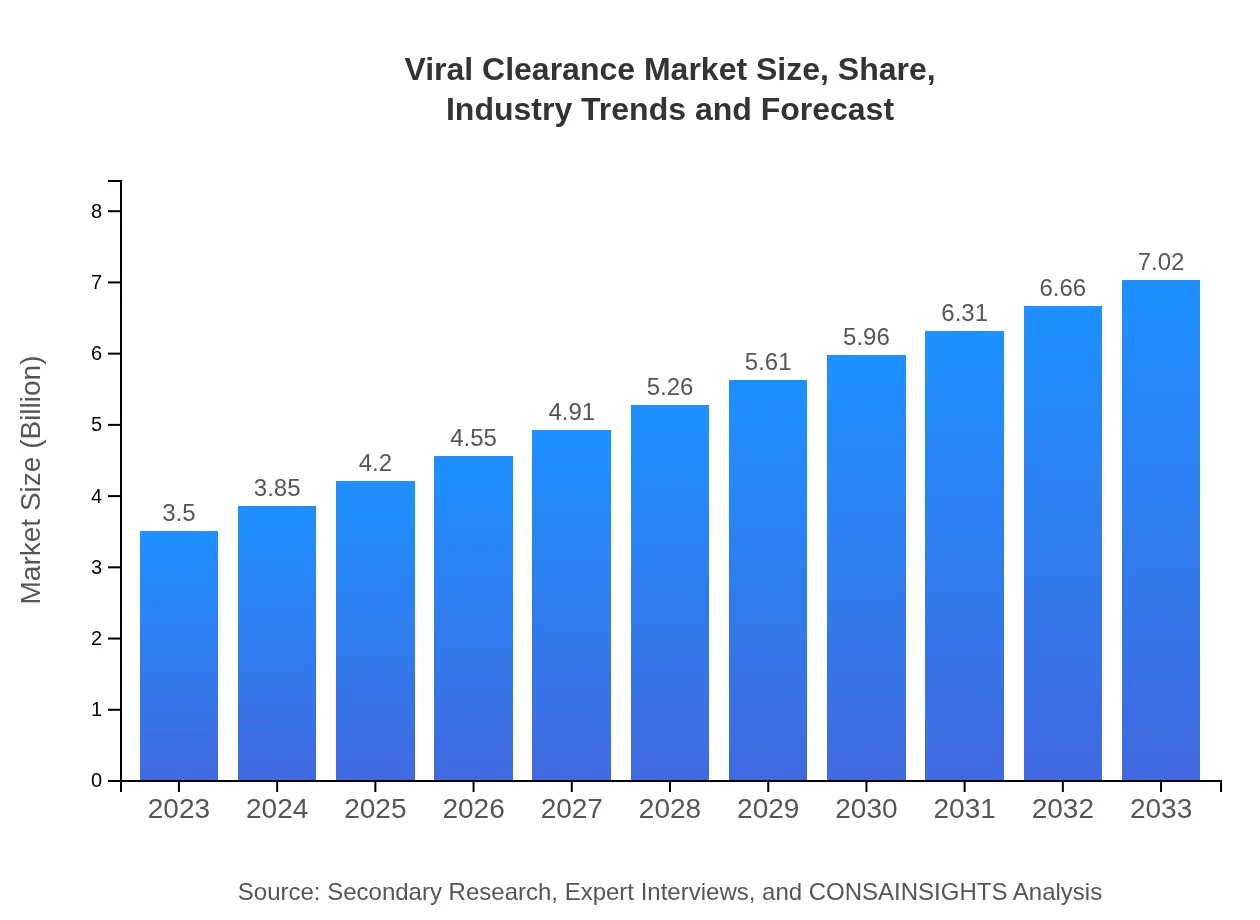
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 7% |
| 2033 Market Size | $7.02 Billion |
| Top Companies | Merck KGaA, Thermo Fisher Scientific, Sartorius AG, Pall Corporation |
| Last Modified Date | 31 January 2026 |
Viral Clearance Market Overview
Customize Viral Clearance Market Report market research report
- ✔ Get in-depth analysis of Viral Clearance market size, growth, and forecasts.
- ✔ Understand Viral Clearance's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Viral Clearance
What is the Market Size & CAGR of Viral Clearance market in 2023?
Viral Clearance Industry Analysis
Viral Clearance Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Viral Clearance Market Analysis Report by Region
Europe Viral Clearance Market Report:
Europe's market is projected to expand from USD 1.11 billion in 2023 to USD 2.22 billion by 2033. Stringent regulatory standards for biopharmaceutical production and heightened focus on product safety drive this growth.Asia Pacific Viral Clearance Market Report:
In Asia Pacific, the Viral Clearance market is poised for growth from USD 0.64 billion in 2023 to USD 1.27 billion in 2033. The region's increasing investments in healthcare infrastructure and a growing number of biopharmaceutical companies drive this expansion.North America Viral Clearance Market Report:
North America remains a key market, with an estimated size of USD 1.26 billion in 2023 and expected to reach USD 2.52 billion by 2033. The presence of numerous established pharmaceutical companies and strong regulatory frameworks underpin its growth.South America Viral Clearance Market Report:
The South American market is expected to grow from USD 0.09 billion in 2023 to USD 0.17 billion by 2033. Developments in healthcare policies and increased focus on viral safety standards are likely contributing factors.Middle East & Africa Viral Clearance Market Report:
The Middle East and Africa region shows growth from USD 0.42 billion in 2023 to USD 0.83 billion in 2033. Increasing awareness of safety protocols in biopharma processes enhances demand for viral clearance solutions.Tell us your focus area and get a customized research report.
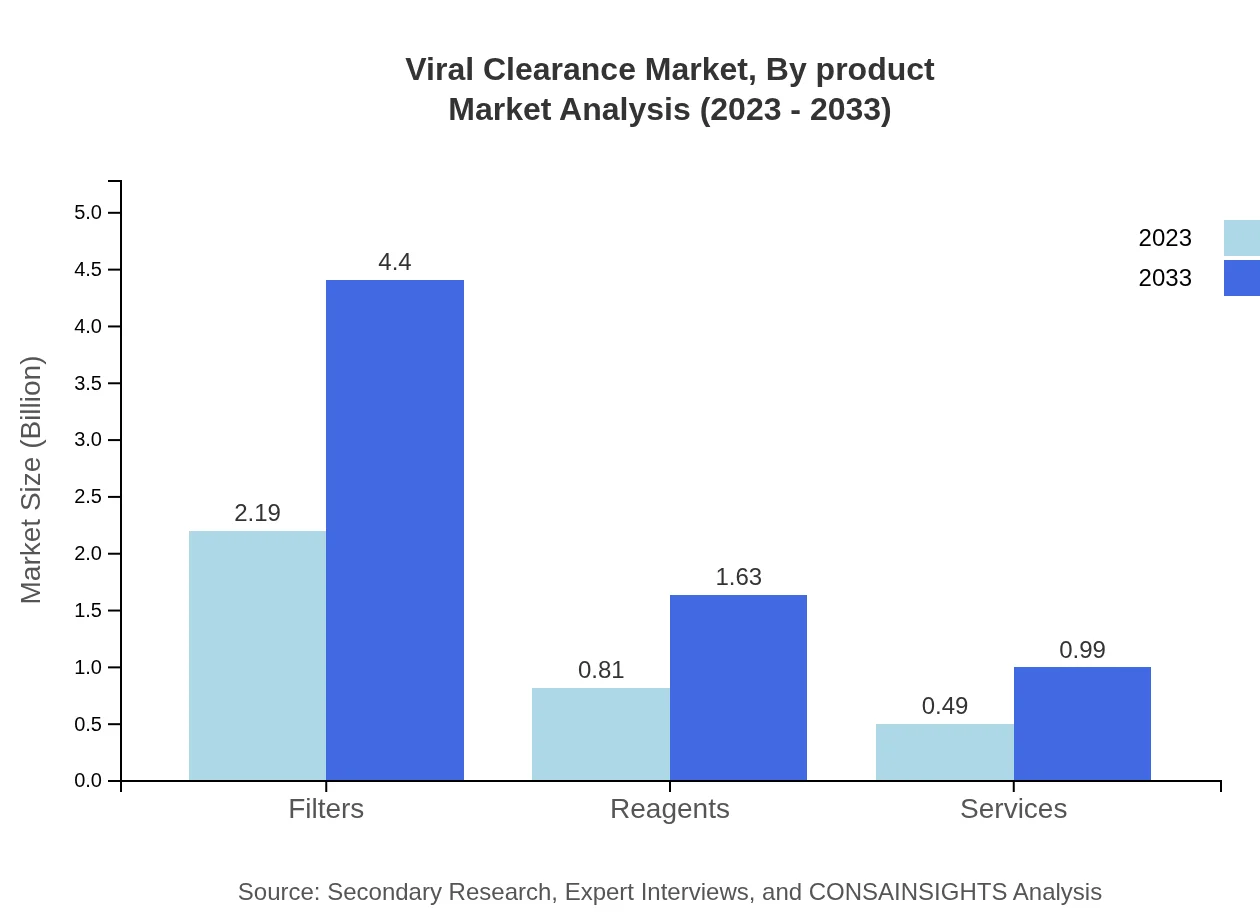
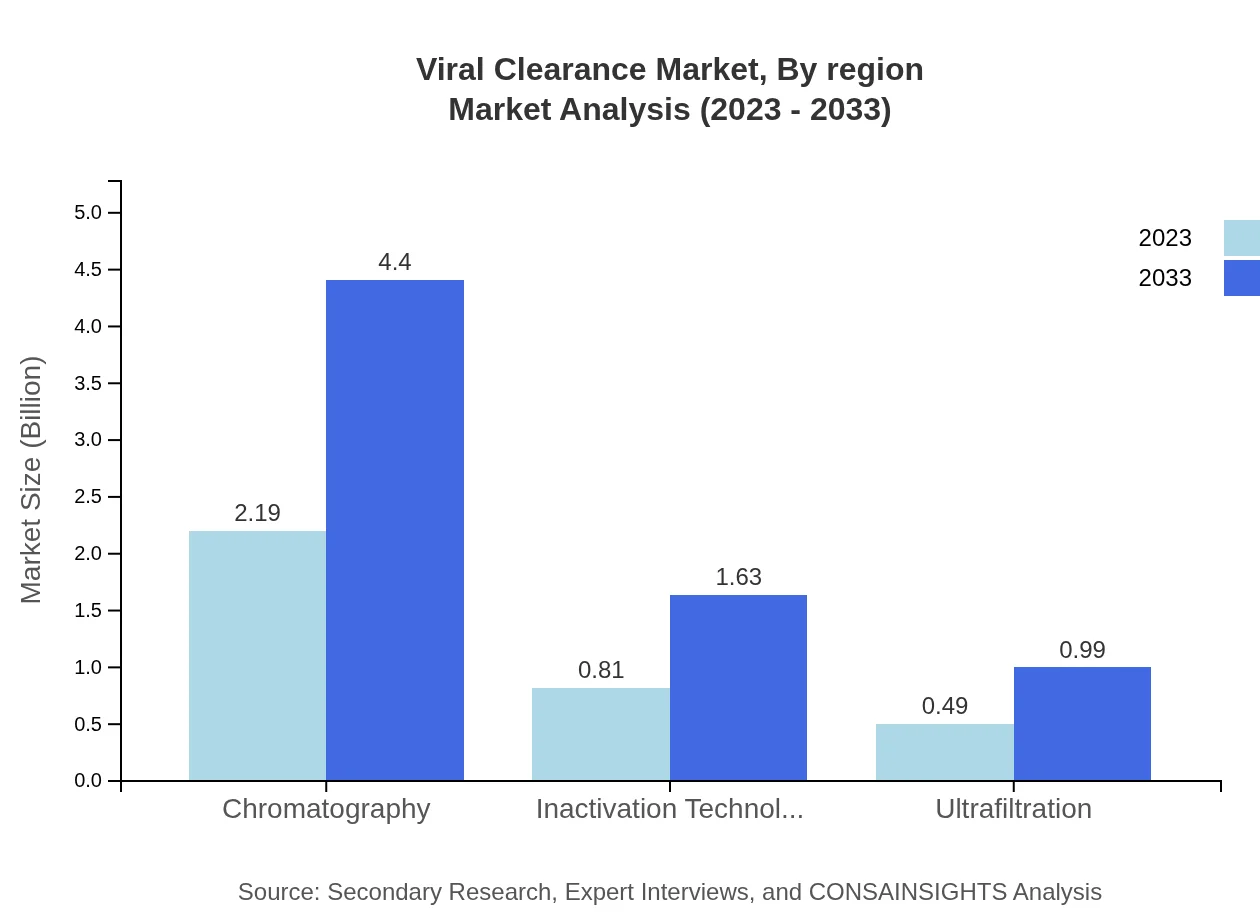
Viral Clearance Market Analysis By Product
The product segment includes major technologies such as Chromatography, Inactivation Technology, Ultrafiltration, Filters, and Reagents. Among these, chromatography is anticipated to hold the largest share, accommodating significant market activity due to its high effectiveness in viral removal. For example, the chromatography market is projected to grow from USD 2.19 billion in 2023 to USD 4.40 billion by 2033, constituting approximately 62.71% of the market share.
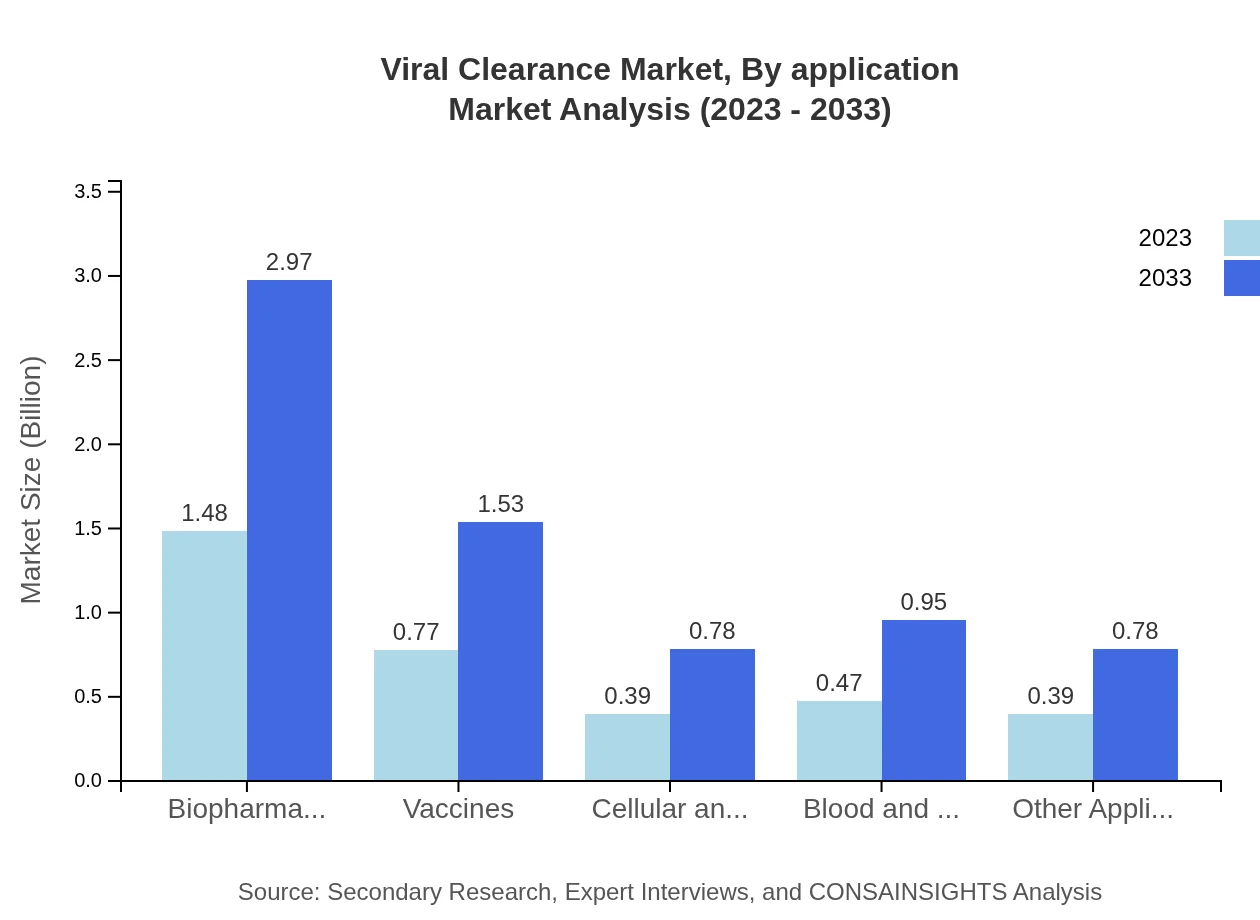
Viral Clearance Market Analysis By Application
This segment highlights applications like biopharmaceuticals, vaccines, and cell and gene therapies. Biopharmaceuticals dominate the marketplace with a significant share of 42.32% in 2023, expected to grow to 42.32% by 2033, backed by the need for rigorous viral safety measures.
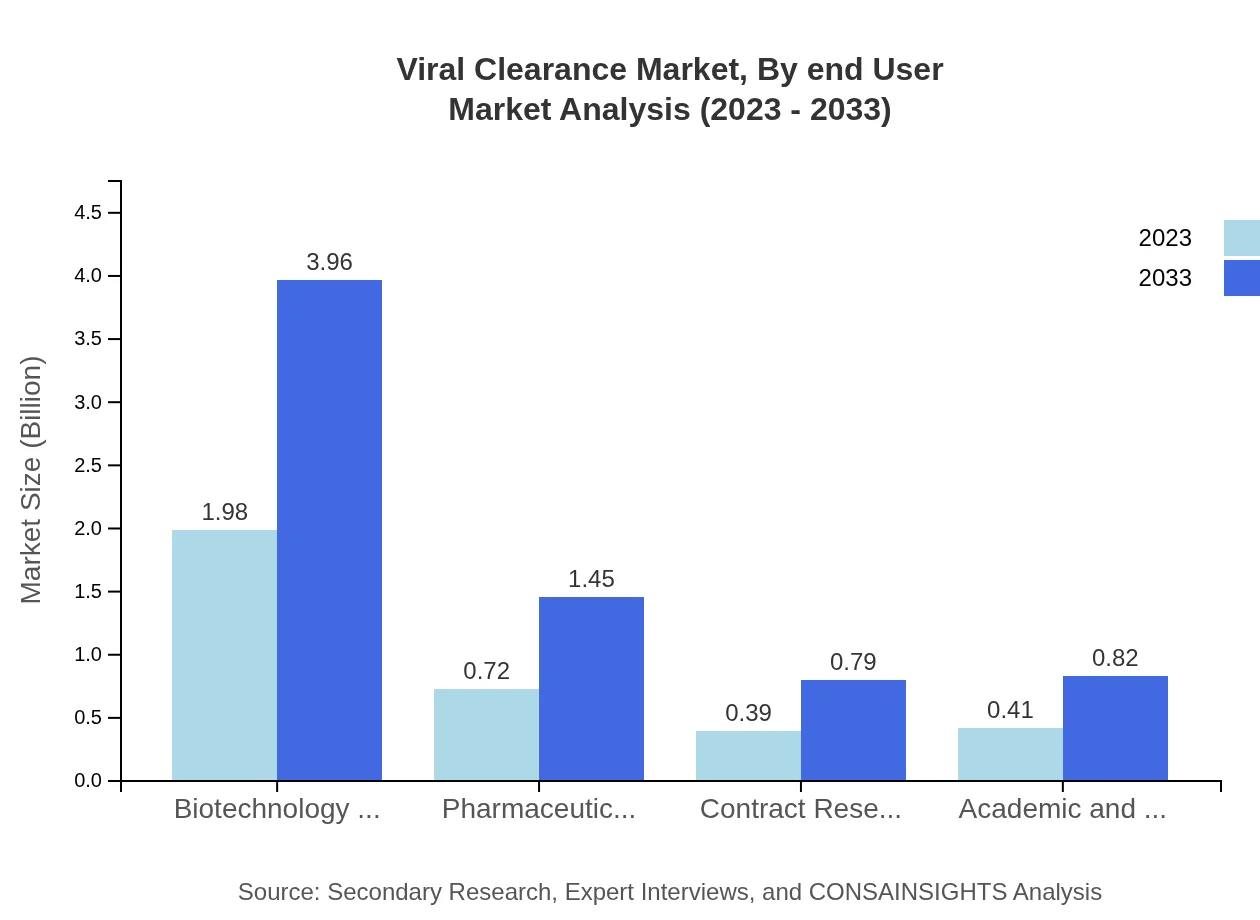
Viral Clearance Market Analysis By End User
End-users include biotechnology companies, pharmaceuticals, and academic and research institutions. Biotechnology companies lead the segment with a market size of USD 1.98 billion in 2023 and are projected to steadily increase their dominance through 2033.
Viral Clearance Market Analysis By Region
This section evaluates market technology trends, including advancements in filtration technologies and chromatography innovations that are shaping the sector's future. Each technology's efficiency and reliability in removing viral contaminants remains a focal point for market players.
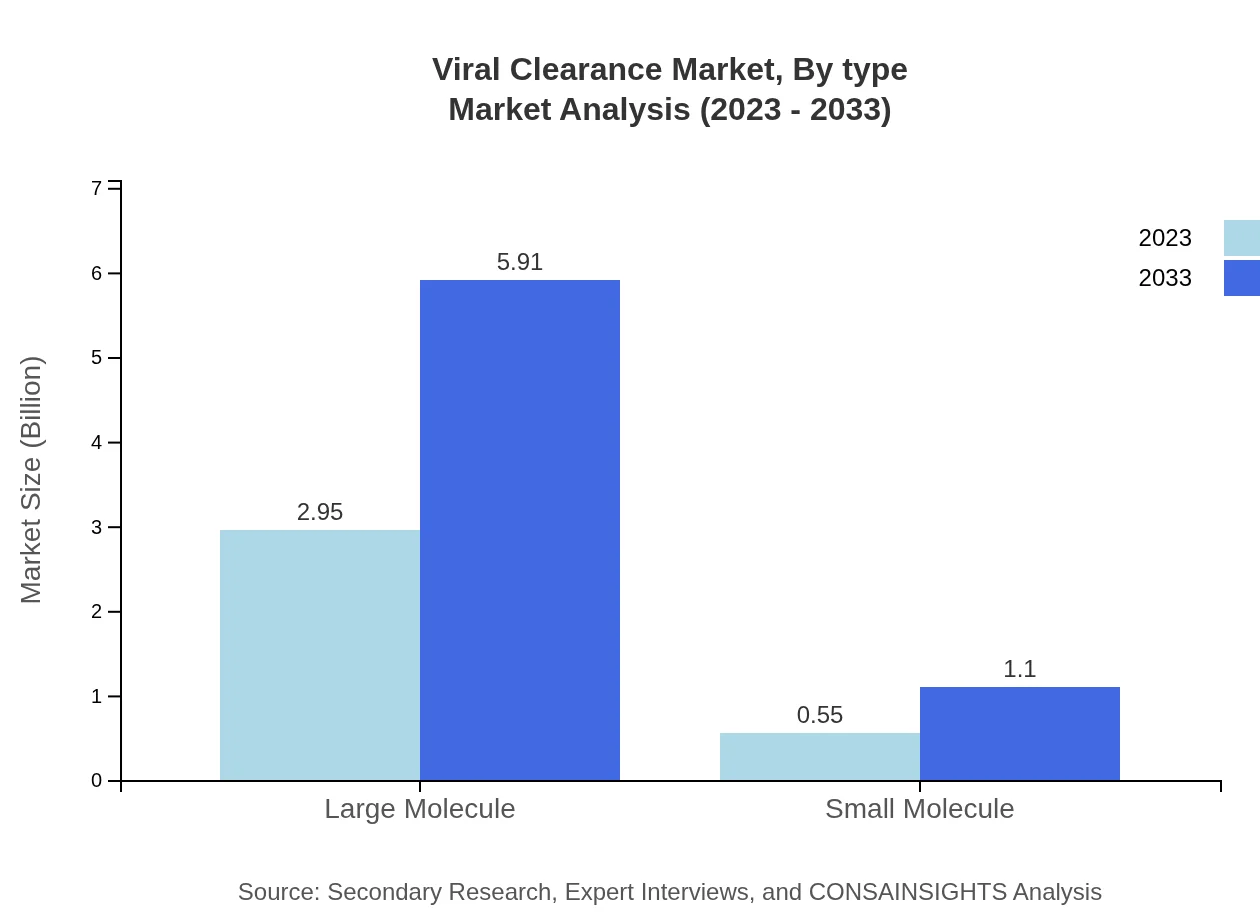
Viral Clearance Market Analysis By Type
The viral clearance market is segmented into technologies, including filtration, chromatography, and various inactivation methods. Each type's growth trajectory will be influenced by factors such as regulatory requirements and clinical demand for safety and efficacy.
Viral Clearance Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Viral Clearance Industry
Merck KGaA:
A leading player in the life sciences sector, Merck KGaA offers various viral clearance solutions and technologies essential for biopharmaceutical manufacturing.Thermo Fisher Scientific:
A global leader in serving science, Thermo Fisher provides comprehensive solutions for viral clearance, including advanced filtration and chromatography products.Sartorius AG:
Sartorius specializes in biotechnology and pharmaceutical industries, offering innovative viral clearance technologies ensuring product safety and quality.Pall Corporation:
Pall Corporation is renowned for its filtration systems and offers specialized solutions for effective viral clearance, targeting the needs of diverse industries.We're grateful to work with incredible clients.









FAQs
What is the market size of viral Clearance?
The viral clearance market is valued at $3.5 billion in 2023, with a projected CAGR of 7% up to 2033. This growth highlights the rising demand for safe and effective viral clearance solutions in the biopharmaceutical and biotechnology sectors.
What are the key market players or companies in this viral Clearance industry?
Key players in the viral clearance industry include major biotechnology companies, pharmaceutical companies, and specialized contract research organizations. Each entity plays a crucial role in developing innovative viral clearance technologies essential for ensuring product safety.
What are the primary factors driving the growth in the viral Clearance industry?
The growth of the viral clearance market is primarily driven by increasing regulatory scrutiny, the rise in biopharmaceutical products, and advances in viral clearance technologies that enhance safety and efficiency in pharmaceutical manufacturing processes.
Which region is the fastest Growing in the viral Clearance?
The fastest-growing region in the viral clearance market is Europe, projected to grow from $1.11 billion in 2023 to $2.22 billion by 2033. This growth is fueled by increased funding in biopharmaceutical R&D and stringent regulatory requirements.
Does ConsaInsights provide customized market report data for the viral Clearance industry?
Yes, ConsaInsights specializes in providing customized market report data tailored to the viral clearance industry. This includes detailed insights on market trends, competitive analysis, and specific regional market dynamics.
What deliverables can I expect from this viral Clearance market research project?
From a viral clearance market research project, you can expect comprehensive reports including market size forecasts, competitive landscape analysis, insights into emerging trends, key player profiles, and segmented data based on applications and technologies.
What are the market trends of viral Clearance?
Current trends in the viral clearance market include the development of more efficient clearance technologies, a focus on regulatory compliance, and increased collaborations between biopharmaceutical companies and CROs to enhance viral safety in products.