Viral Inactivation Market Report
Published Date: 31 January 2026 | Report Code: viral-inactivation
Viral Inactivation Market Size, Share, Industry Trends and Forecast to 2033
This report covers a comprehensive analysis of the viral inactivation market from 2023 to 2033, providing insights into market trends, size, segmentation, regional dynamics, key players, and future forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
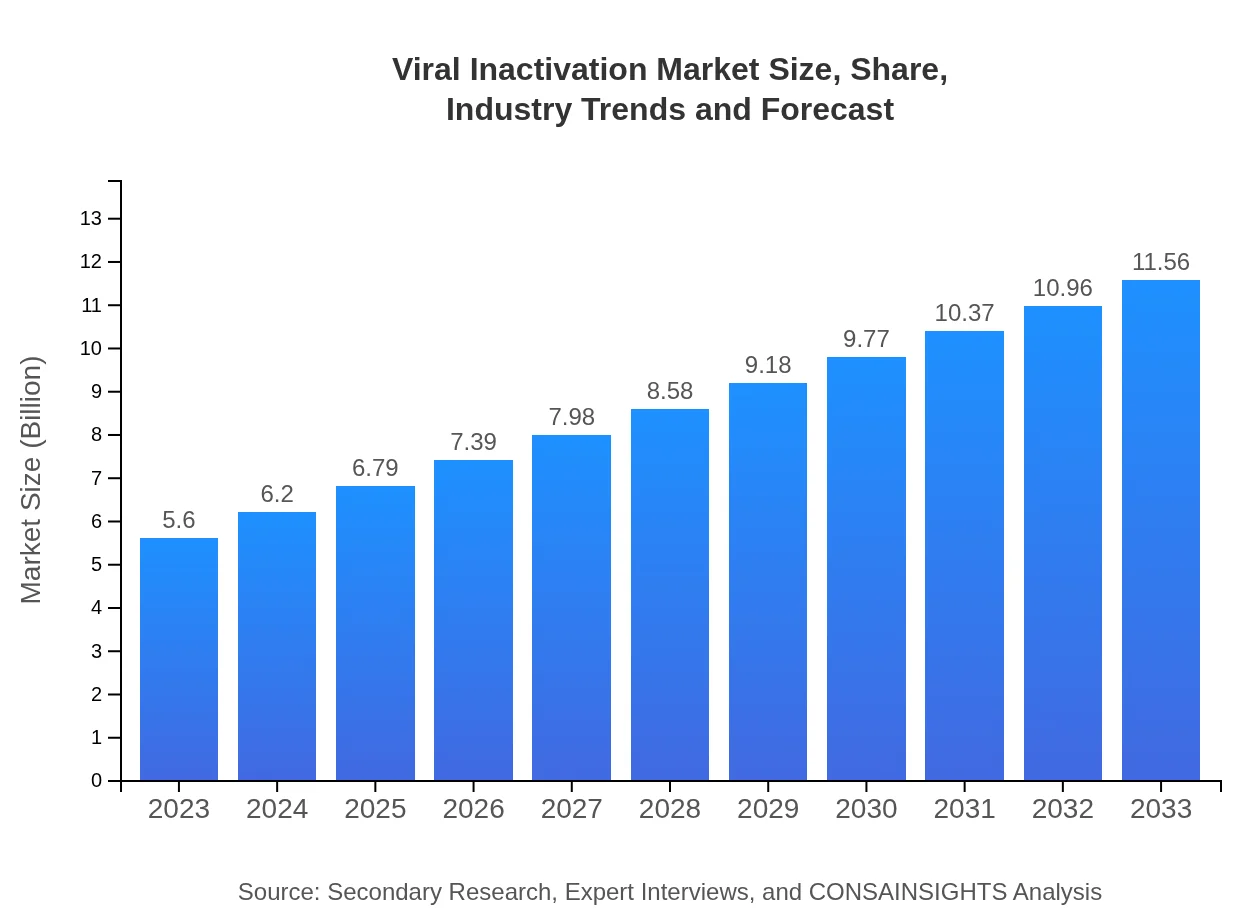
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.3% |
| 2033 Market Size | $11.56 Billion |
| Top Companies | Thermo Fisher Scientific, Merck KGaA, Charles River Laboratories, Sartorius AG, Pall Corporation |
| Last Modified Date | 31 January 2026 |
Viral Inactivation Market Overview
Customize Viral Inactivation Market Report market research report
- ✔ Get in-depth analysis of Viral Inactivation market size, growth, and forecasts.
- ✔ Understand Viral Inactivation's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Viral Inactivation
What is the Market Size & CAGR of Viral Inactivation market in 2023?
Viral Inactivation Industry Analysis
Viral Inactivation Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Viral Inactivation Market Analysis Report by Region
Europe Viral Inactivation Market Report:
Europe shows substantial growth, expected to move from $1.39 billion in 2023 to approximately $2.87 billion by 2033. This growth is influenced by stringent regulations regarding product safety, coupled with a growing adoption of viral inactivation technologies across various applications in pharmaceuticals and healthcare.Asia Pacific Viral Inactivation Market Report:
The Asia Pacific region is forecasted to grow significantly from a market size of $1.12 billion in 2023 to approximately $2.31 billion by 2033. The growth is fueled by increasing healthcare investments, advancements in drug manufacturing processes, and rising demand for safe biopharmaceuticals driven by regulatory compliance and awareness of viral safety.North America Viral Inactivation Market Report:
North America leads the viral inactivation market with a projected size expansion from $2.03 billion in 2023 to $4.19 billion by 2033. The region's growth is supported by a robust framework of regulations, high investments in research and development, and the significant presence of leading market players who drive innovation and technology adoption.South America Viral Inactivation Market Report:
In South America, the market is expected to see growth from $0.39 billion in 2023 to about $0.81 billion by 2033, primarily due to improvements in healthcare infrastructure and the expansion of the biopharmaceutical sector. Increased regulatory focus on product safety also plays a crucial role in driving market growth in this region.Middle East & Africa Viral Inactivation Market Report:
The Middle East and Africa's market is anticipated to grow from $0.67 billion in 2023 to $1.38 billion by 2033, driven by improvements in healthcare services and rising awareness regarding viral safety in the production of biological products.Tell us your focus area and get a customized research report.
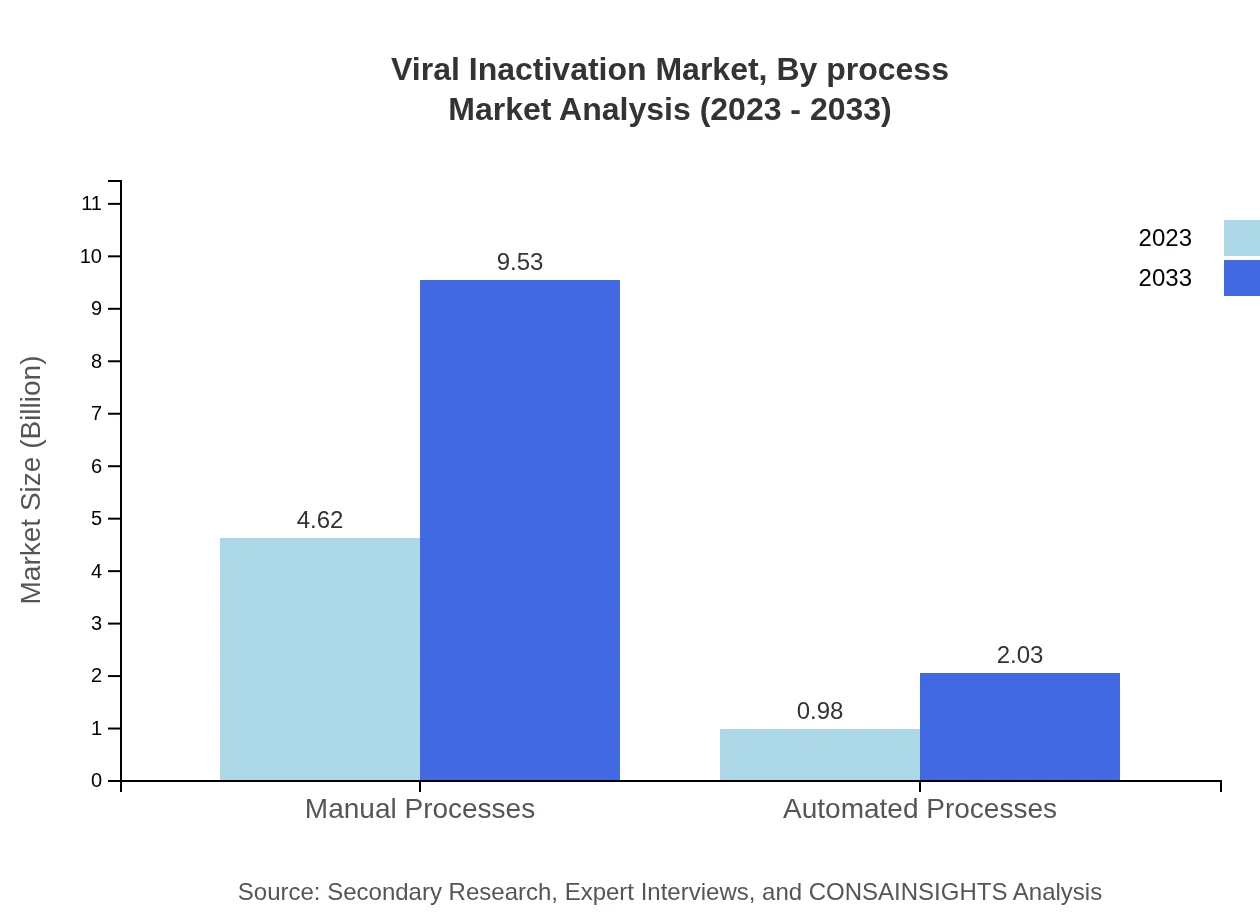
Viral Inactivation Market Analysis By Technology
The technology segment of the viral inactivation market is characterized by manual and automated processes. In 2023, the market for manual processes is projected to be valued at $4.62 billion, maintaining an 82.42% share, while automated processes are valued at $0.98 billion with a 17.58% share. By 2033, values are expected to rise to $9.53 billion and $2.03 billion respectively, reflecting a shift towards automation aimed at improving efficiency and reducing human error.
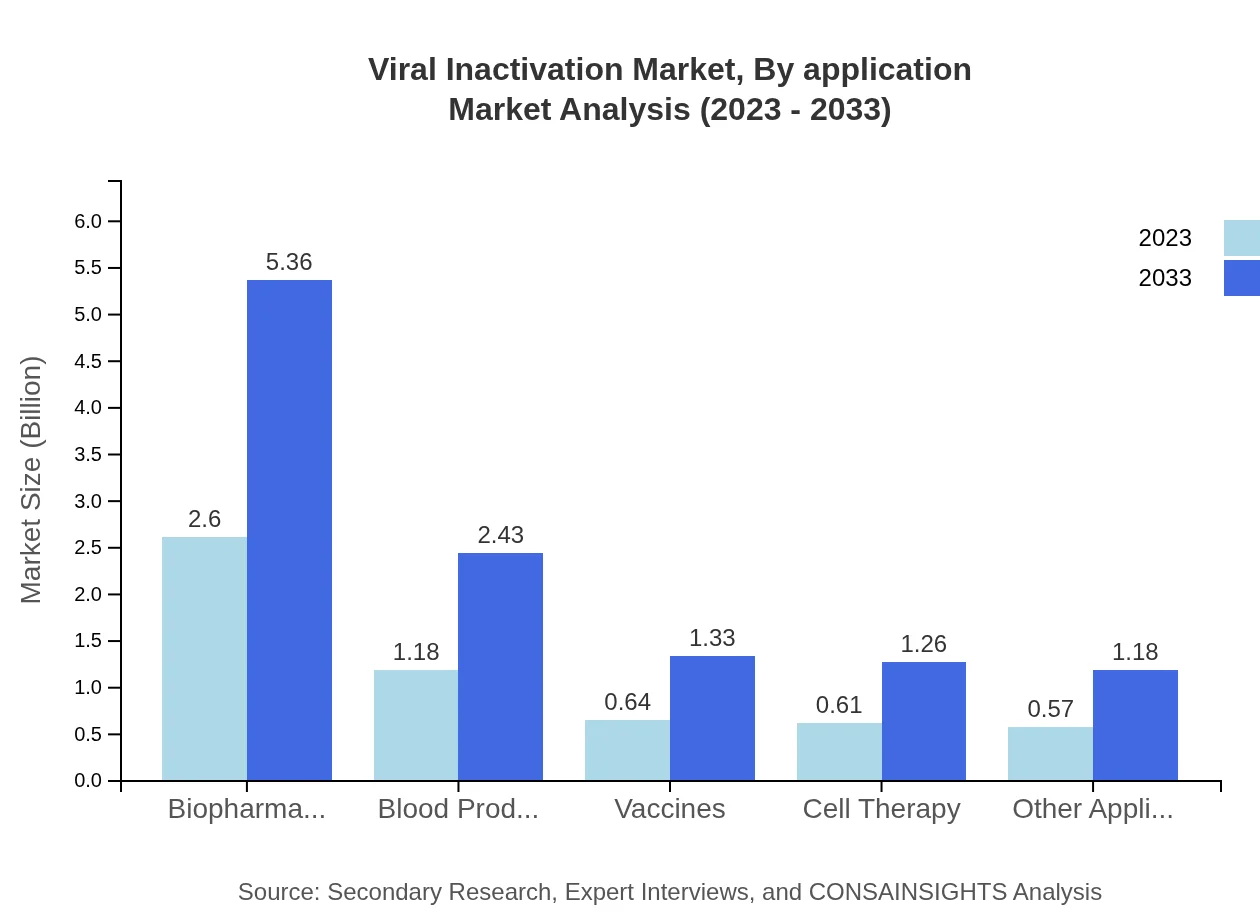
Viral Inactivation Market Analysis By Application
The application analysis indicates that the market is segmented primarily into blood products, biopharmaceuticals, vaccines, and others. Blood products contribute a significant share of the market with a size projected at $1.18 billion in 2023, growing to $2.43 billion by 2033, while biopharmaceuticals are expected to rise from $2.60 billion to $5.36 billion during the same period.
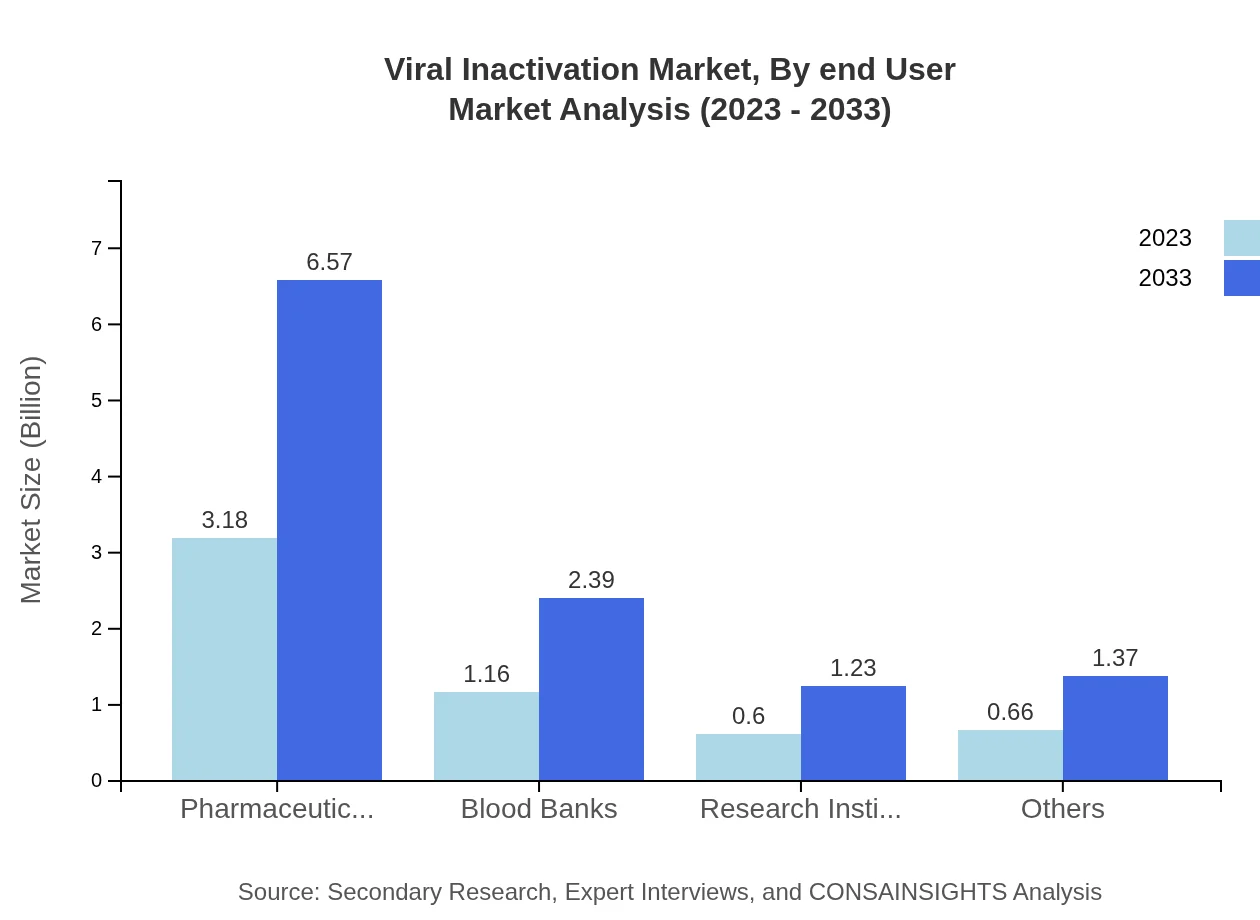
Viral Inactivation Market Analysis By End User
End-users include pharmaceutical companies, blood banks, and research institutes. Pharmaceutical companies are projected to dominate the market with a size of $3.18 billion in 2023, growing to $6.57 billion by 2033. Blood banks and research institutes are also significant contributors, with respective market sizes of $1.16 billion and $0.60 billion, both demonstrating growth as viral safety concerns mount.
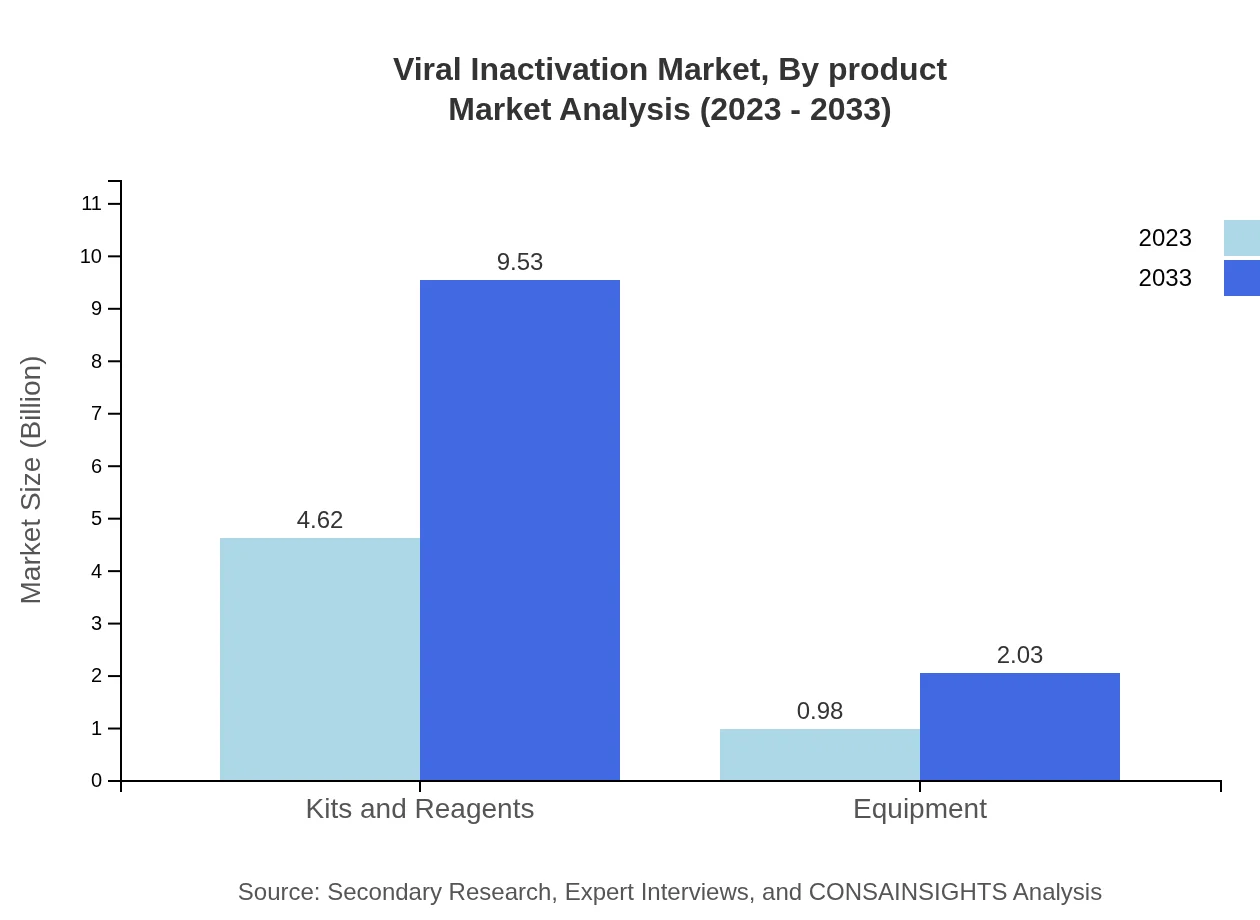
Viral Inactivation Market Analysis By Product
Different product types such as kits, reagents, and equipment play crucial roles in the market. In 2023, the market for kits and reagents is anticipated to be around $4.62 billion, whereas equipment will account for $0.98 billion. By 2033 the expected sizes for both are projected to reach $9.53 billion and $2.03 billion respectively, indicating a solid demand for both across various applications.
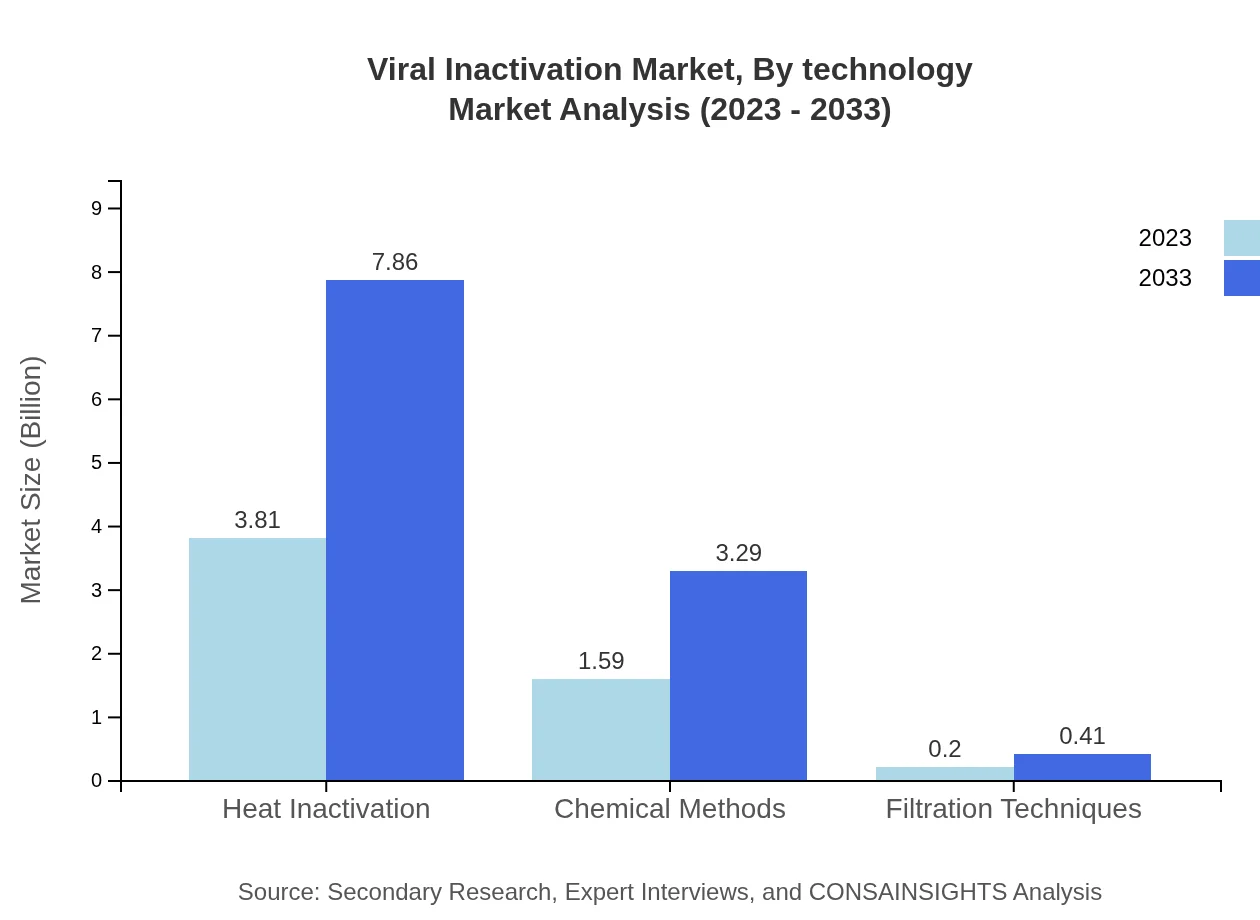
Viral Inactivation Market Analysis By Process
The process segment analyses different methods such as heat inactivation, chemical methods, and filtration techniques. Heat inactivation remains dominant with a size of $3.81 billion in 2023, expected to rise to $7.86 billion by 2033, driven by its proven effectiveness and widespread application. Conversely, chemical methods are projected to grow from $1.59 billion to $3.29 billion, highlighting innovation in diverse chemical processes.
Viral Inactivation Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Viral Inactivation Industry
Thermo Fisher Scientific:
A leader in scientific instruments and laboratory equipment, Thermo Fisher provides innovative solutions in viral inactivation technologies, contributing significantly to biopharmaceutical safety.Merck KGaA:
Merck KGaA offers a broad array of bioprocessing solutions including viral inactivation services to enhance product safety across multiple applications, strengthening their market position.Charles River Laboratories:
Specializing in products and services to support drug development, Charles River Laboratories provides reputable viral inactivation services essential for the safety of biological therapies.Sartorius AG:
Sartorius AG is recognized for its advancements in bioprocessing and technology solutions, significantly impacting the efficiency of viral inactivation in biomanufacturing.Pall Corporation:
Pall Corporation specializes in filtration and separation technologies, offering robust viral inactivation solutions crucial for maintaining product integrity and compliance with safety standards.We're grateful to work with incredible clients.









FAQs
What is the market size of viral Inactivation?
The global viral inactivation market is valued at approximately $5.6 billion in 2023. With a projected CAGR of 7.3%, it is set to reach new heights by 2033, ensuring significant growth in this crucial sector.
What are the key market players or companies in this industry?
Key players in the viral inactivation market include prominent pharmaceutical firms and biotechnology companies focused on enhancing safety measures. These industry leaders are continually innovating to meet regulatory standards, ensuring effective viral inactivation processes for various applications.
What are the primary factors driving the growth in the viral inactivation industry?
The viral inactivation market is primarily driven by the increasing demand for safety in biologics production, stringent regulatory requirements, and the rise in viral outbreaks. Additionally, advancements in technology and a growing focus on biopharmaceuticals further fuel market expansion.
Which region is the fastest Growing in the viral inactivation market?
Among various regions, North America currently leads the viral inactivation market. However, the Asia Pacific region is expected to exhibit rapid growth, projected to increase from $1.12 billion in 2023 to $2.31 billion by 2033, indicating a strong upward trend.
Does ConsaInsights provide customized market report data for the viral inactivation industry?
Yes, ConsaInsights offers tailored market report data for the viral inactivation industry. Clients can benefit from customized analyses, including specific geographical and segment-focused insights tailored to their unique business needs.
What deliverables can I expect from this viral inactivation market research project?
Clients can expect comprehensive deliverables, including detailed market analysis, growth forecasts, competitive landscape evaluations, and insights into key trends and challenges in the viral inactivation market, all presented in an easily digestible format.
What are the market trends of viral inactivation?
Current trends in the viral inactivation market indicate a shift toward automated processes and innovative technologies. The increasing reliance on effective and reliable inactivation methods underscores the industry's ongoing evolution, reflecting enhanced safety measures in the healthcare sector.