Virtual Clinical Trials Market Report
Published Date: 31 January 2026 | Report Code: virtual-clinical-trials
Virtual Clinical Trials Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Virtual Clinical Trials market, including market size, trends, and forecasts up to 2033. It covers critical insights into industry segments, regional dynamics, and technological developments shaping future growth.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
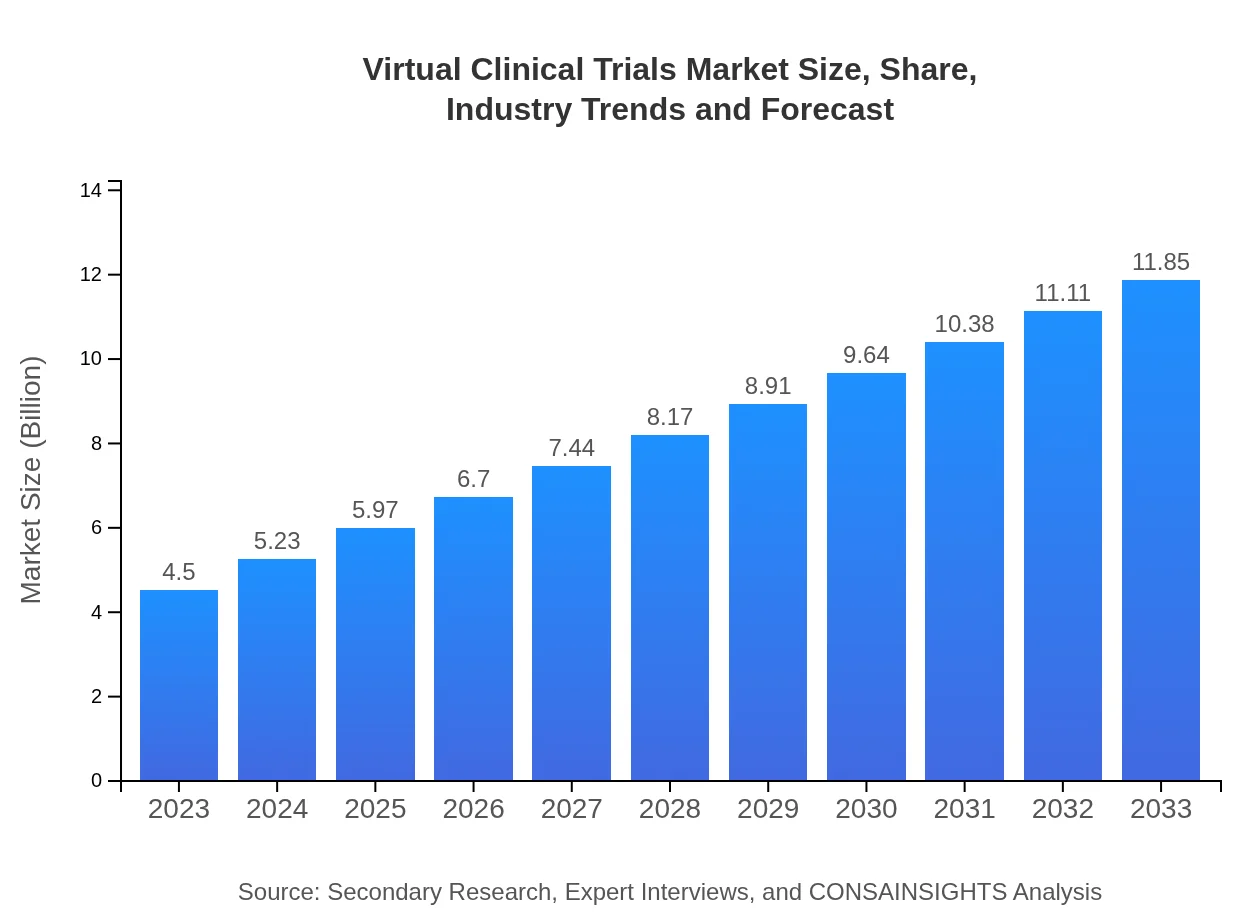
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 9.8% |
| 2033 Market Size | $11.85 Billion |
| Top Companies | Medidata Solutions, Parexel International, Covance |
| Last Modified Date | 31 January 2026 |
Virtual Clinical Trials Market Overview
Customize Virtual Clinical Trials Market Report market research report
- ✔ Get in-depth analysis of Virtual Clinical Trials market size, growth, and forecasts.
- ✔ Understand Virtual Clinical Trials's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Virtual Clinical Trials
What is the Market Size & CAGR of Virtual Clinical Trials market in 2023?
Virtual Clinical Trials Industry Analysis
Virtual Clinical Trials Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Virtual Clinical Trials Market Analysis Report by Region
Europe Virtual Clinical Trials Market Report:
Europe's market size was approximately $1.39 billion in 2023, projected to grow to $3.65 billion by 2033. The region is at the forefront of adopting digital health solutions, backed by robust regulatory frameworks that facilitate virtual trials.Asia Pacific Virtual Clinical Trials Market Report:
The Asia Pacific region is witnessing significant growth in the Virtual Clinical Trials market, with a market size estimated at $0.76 billion in 2023, projected to reach $2.00 billion by 2033. The increasing acceptance of digital technologies in healthcare, coupled with a large patient population, serves as a driving force.North America Virtual Clinical Trials Market Report:
North America dominates the Virtual Clinical Trials market, with a 2023 valuation of $1.69 billion, expected to rise to $4.46 billion by 2033. The region benefits from advanced healthcare systems, strong technological adoption among stakeholders, and regulatory support for decentralized clinical trials.South America Virtual Clinical Trials Market Report:
In South America, the Virtual Clinical Trials market size was $0.40 billion in 2023, with expectations to grow to $1.05 billion by 2033. The region's growing healthcare infrastructure and focus on improving clinical research methodologies are likely to bolster this growth.Middle East & Africa Virtual Clinical Trials Market Report:
The Virtual Clinical Trials market in the Middle East and Africa was valued at $0.26 billion in 2023, expected to reach $0.68 billion by 2033. Factors driving growth include improved healthcare access and investment in technology to enhance clinical study efficiency.Tell us your focus area and get a customized research report.
Virtual Clinical Trials Market Analysis By Trial Type
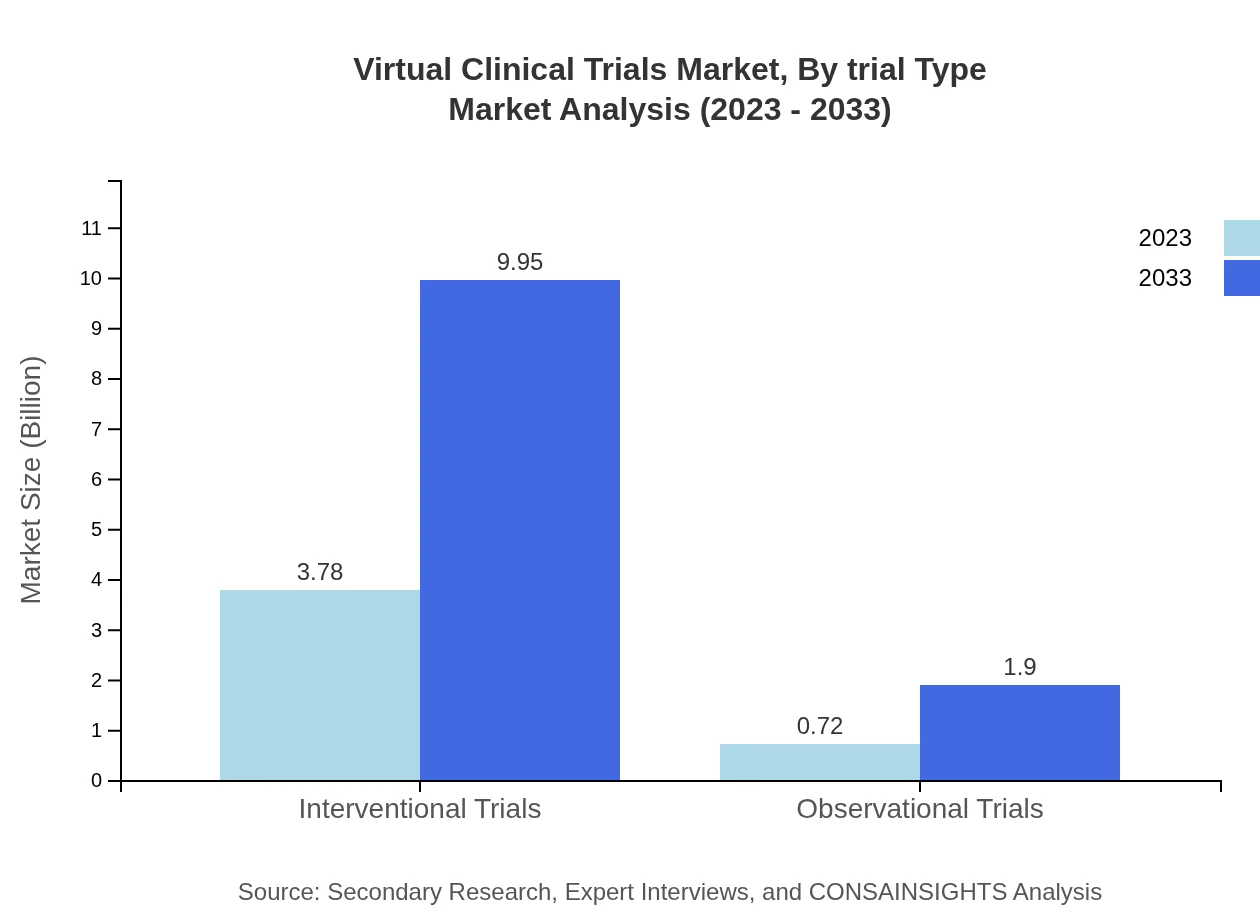
In 2023, the Virtual Clinical Trials market segment by trial type is divided into interventional and observational trials, with interventional trials generating $3.78 billion in size. Their share remains dominant at approximately 83.93%. Observational trials, valued at $0.72 billion and maintaining a 16.07% market share, are also gaining traction as an alternative study method.
Virtual Clinical Trials Market Analysis By Technology
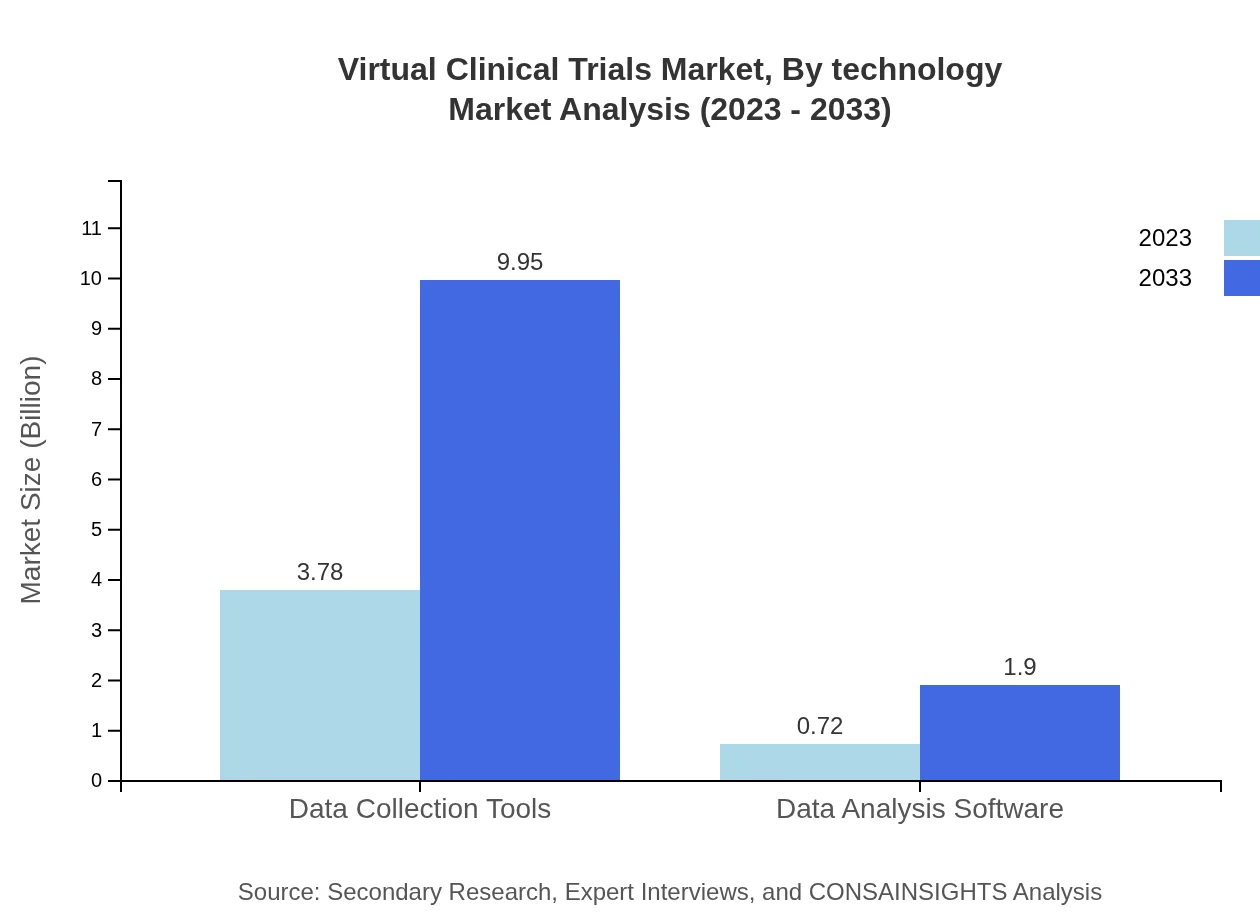
Technology plays a pivotal role in enhancing the execution of Virtual Clinical Trials. As of 2023, Data Collection Tools are leading the segment with a market size of $3.78 billion, maintaining an 83.93% share. Data Analysis Software, with a size of $0.72 billion and 16.07% share, also proves crucial in facilitating data-driven decision-making.
Virtual Clinical Trials Market Analysis By Phase
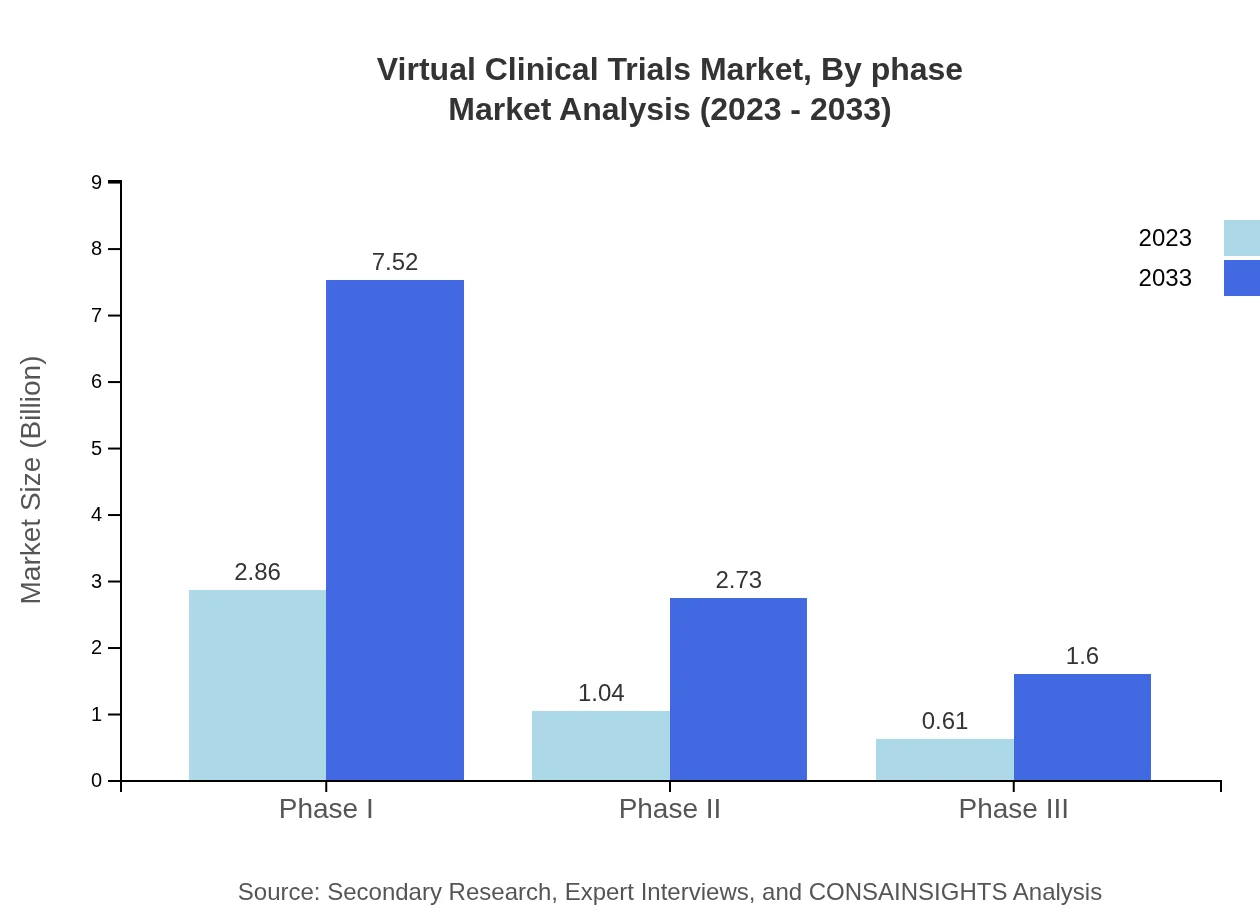
The market analysis by phase reveals a strong performance in Phase I trials, valued at $2.86 billion in 2023, projected to grow to $7.52 billion by 2033. Phase II trials are also notable, growing from $1.04 billion to $2.73 billion, while Phase III trials are expected to rise from $0.61 billion to $1.60 billion during the same period.
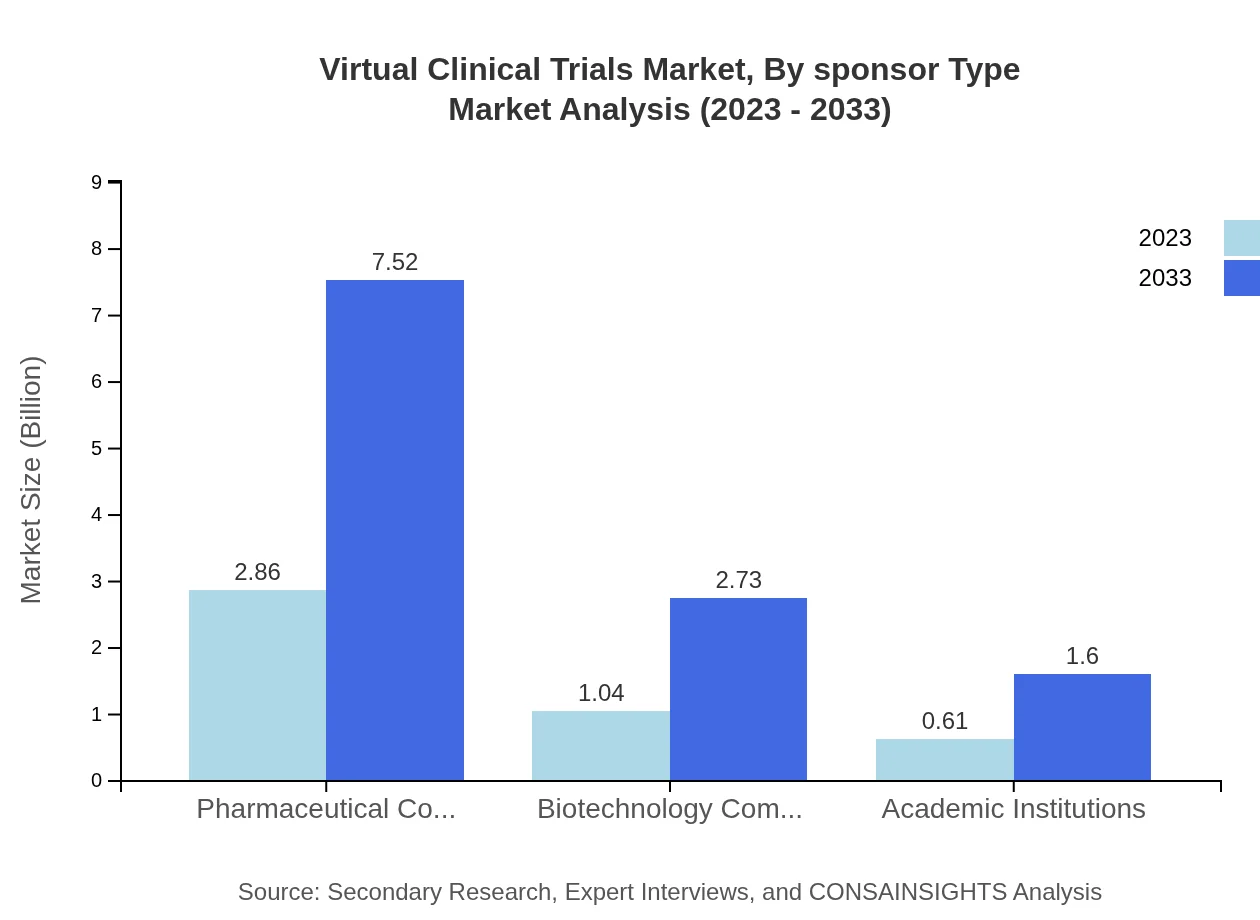
Virtual Clinical Trials Market Analysis By Sponsor Type
Pharmaceutical companies account for the largest share in Virtual Clinical Trials, with a size of $2.86 billion (63.50%) in 2023, expected to grow to $7.52 billion by 2033. Biotechnology companies follow, increasing from $1.04 billion (23.01%) to $2.73 billion by 2033.
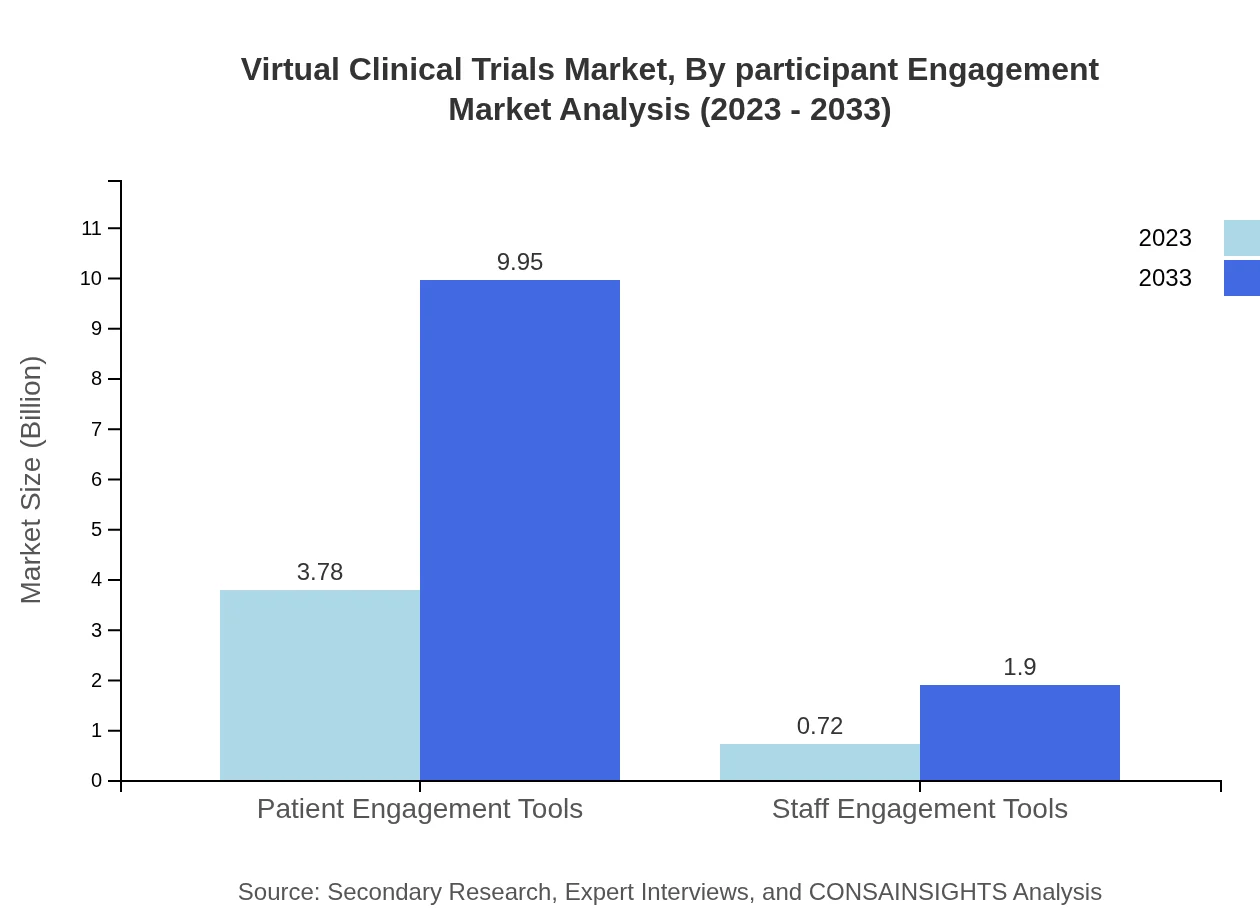
Virtual Clinical Trials Market Analysis By Participant Engagement
With growing emphasis on patient-centric approaches, the market for patient engagement tools is crucial, valued at $3.78 billion in 2023 (83.93%) and expected to grow to $9.95 billion by 2033. Meanwhile, staff engagement tools are anticipated to grow from $0.72 billion (16.07%) to $1.90 billion.
Virtual Clinical Trials Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Virtual Clinical Trials Industry
Medidata Solutions:
A world leader in cloud-based solutions that advance clinical development, Medidata provides technology platforms for streamlining clinical trials.Parexel International:
Parexel is a prominent global provider of biopharmaceutical services, enhancing the efficiency of clinical trials through innovative technologies and solutions.Covance:
Covance, a division of Labcorp, focuses on drug development services, delivering a comprehensive suite of clinical trial solutions to support sponsors.We're grateful to work with incredible clients.









FAQs
What is the market size of virtual Clinical Trials?
The virtual clinical trials market is projected to reach approximately $4.5 billion by 2033, growing at a CAGR of 9.8%. This growth is driven by advancements in technology and the increasing demand for efficient clinical trial methodologies.
What are the key market players or companies in this virtual Clinical Trials industry?
Key players include major pharmaceutical companies and biotechnological firms. The industry is characterized by collaboration among innovative startups and established corporations aiming to enhance clinical trial efficiency and effectiveness.
What are the primary factors driving the growth in the virtual Clinical Trials industry?
Factors driving growth include technological advancements, increased patient engagement, regulatory support, and the rising need for faster clinical trial processes. Additionally, the COVID-19 pandemic accelerated the adoption of virtual trials across the industry.
Which region is the fastest Growing in the virtual Clinical Trials?
Among regions, North America is the fastest-growing, expected to expand from $1.69 billion in 2023 to $4.46 billion by 2033. Europe and Asia Pacific also show significant growth contributing to the global market expansion.
Does ConsaInsights provide customized market report data for the virtual Clinical Trials industry?
Yes, ConsaInsights offers tailored market reports that can be customized to meet the specific needs of clients, providing detailed insights into market trends, competitive landscape, and regional opportunities.
What deliverables can I expect from this virtual Clinical Trials market research project?
Deliverables include comprehensive reports, executive summaries, market forecasts, competitor analysis, and insights into segment-specific data, with a focus on actionable strategies for market penetration and growth.
What are the market trends of virtual Clinical Trials?
Market trends include a shift towards decentralized trials, integration of digital technologies, and increased focus on patient-centric approaches. Data analysis tools and patient engagement technologies are gaining prominence in shaping trial methodologies.