Von Willebrand Disease Treatment Market Report
Published Date: 31 January 2026 | Report Code: von-willebrand-disease-treatment
Von Willebrand Disease Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Von Willebrand Disease treatment market for the forecast period 2023-2033. It encompasses insights into market size, growth trends, regional dynamics, and key players shaping the industry's future.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
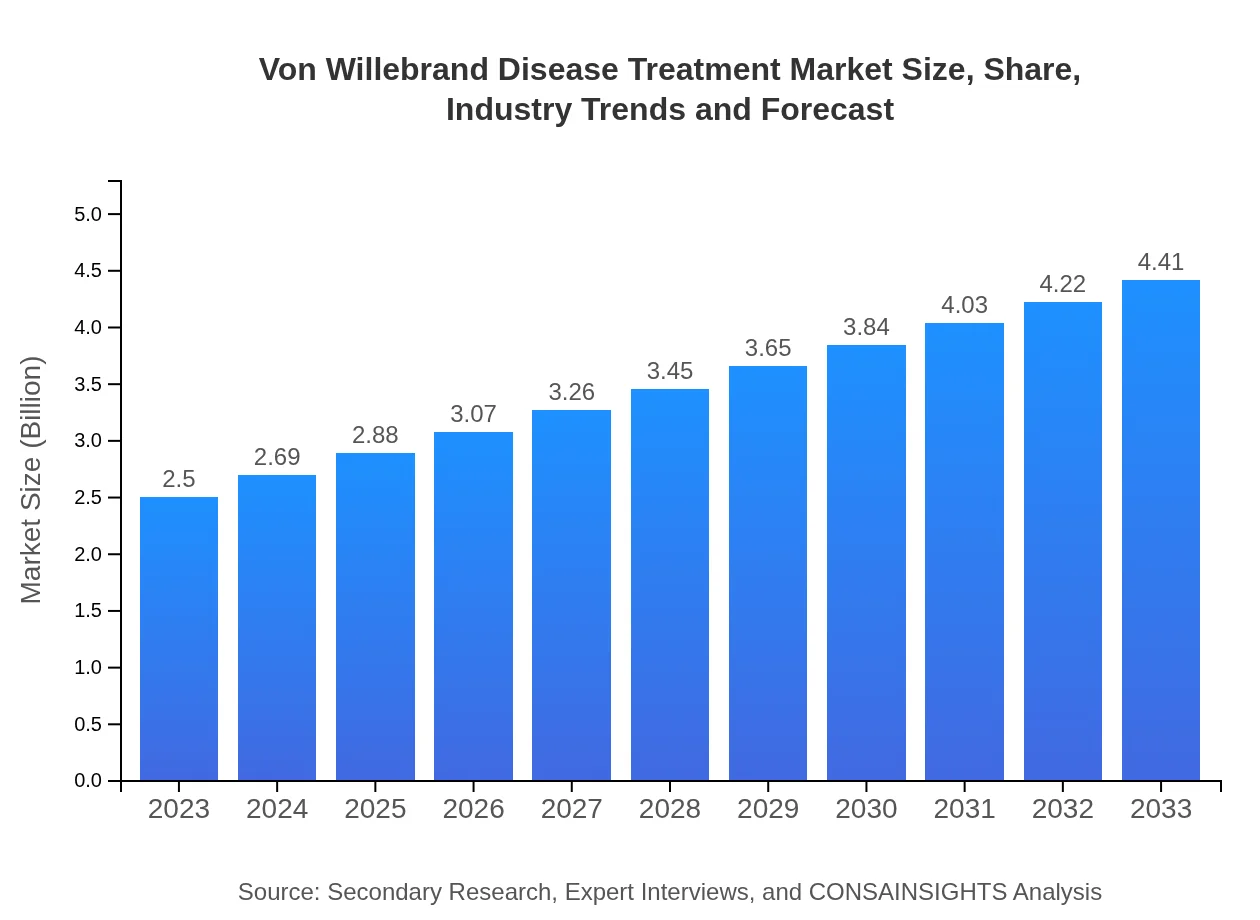
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.7% |
| 2033 Market Size | $4.41 Billion |
| Top Companies | Takeda Pharmaceuticals, Baxter International Inc., CSL Behring, Grifols |
| Last Modified Date | 31 January 2026 |
Von Willebrand Disease Treatment Market Overview
Customize Von Willebrand Disease Treatment Market Report market research report
- ✔ Get in-depth analysis of Von Willebrand Disease Treatment market size, growth, and forecasts.
- ✔ Understand Von Willebrand Disease Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Von Willebrand Disease Treatment
What is the Market Size & CAGR of Von Willebrand Disease Treatment market in 2023?
Von Willebrand Disease Treatment Industry Analysis
Von Willebrand Disease Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Von Willebrand Disease Treatment Market Analysis Report by Region
Europe Von Willebrand Disease Treatment Market Report:
In Europe, the market size was approximately $0.78 billion in 2023, projected to grow to $1.37 billion by 2033. The rise in VWD diagnoses, along with government initiatives promoting blood disorder awareness, aids this market expansion.Asia Pacific Von Willebrand Disease Treatment Market Report:
The Asia Pacific region's Von Willebrand Disease treatment market was valued at $0.52 billion in 2023 and is expected to reach $0.92 billion in 2033. The growth is driven by rising awareness initiatives, improved healthcare access, and increased research funding in countries like Japan and India.North America Von Willebrand Disease Treatment Market Report:
North America leads the market, valued at $0.82 billion in 2023, with projections to hit $1.44 billion in 2033. The strong presence of key market players, advanced healthcare technologies, and increasing patient advocacy efforts significantly drive this growth.South America Von Willebrand Disease Treatment Market Report:
South America's market was valued at $0.22 billion in 2023 and is projected to grow to $0.40 billion by 2033. Expanding healthcare infrastructures and a focus on enhancing treatment methodologies contribute to regional growth.Middle East & Africa Von Willebrand Disease Treatment Market Report:
The market for the Middle East and Africa was valued at $0.16 billion in 2023 and is expected to grow to $0.29 billion by 2033. Improving healthcare facilities and a focus on hemophilia and VWD treatments support growth in this region.Tell us your focus area and get a customized research report.
Von Willebrand Disease Treatment Market Analysis By Treatment Type
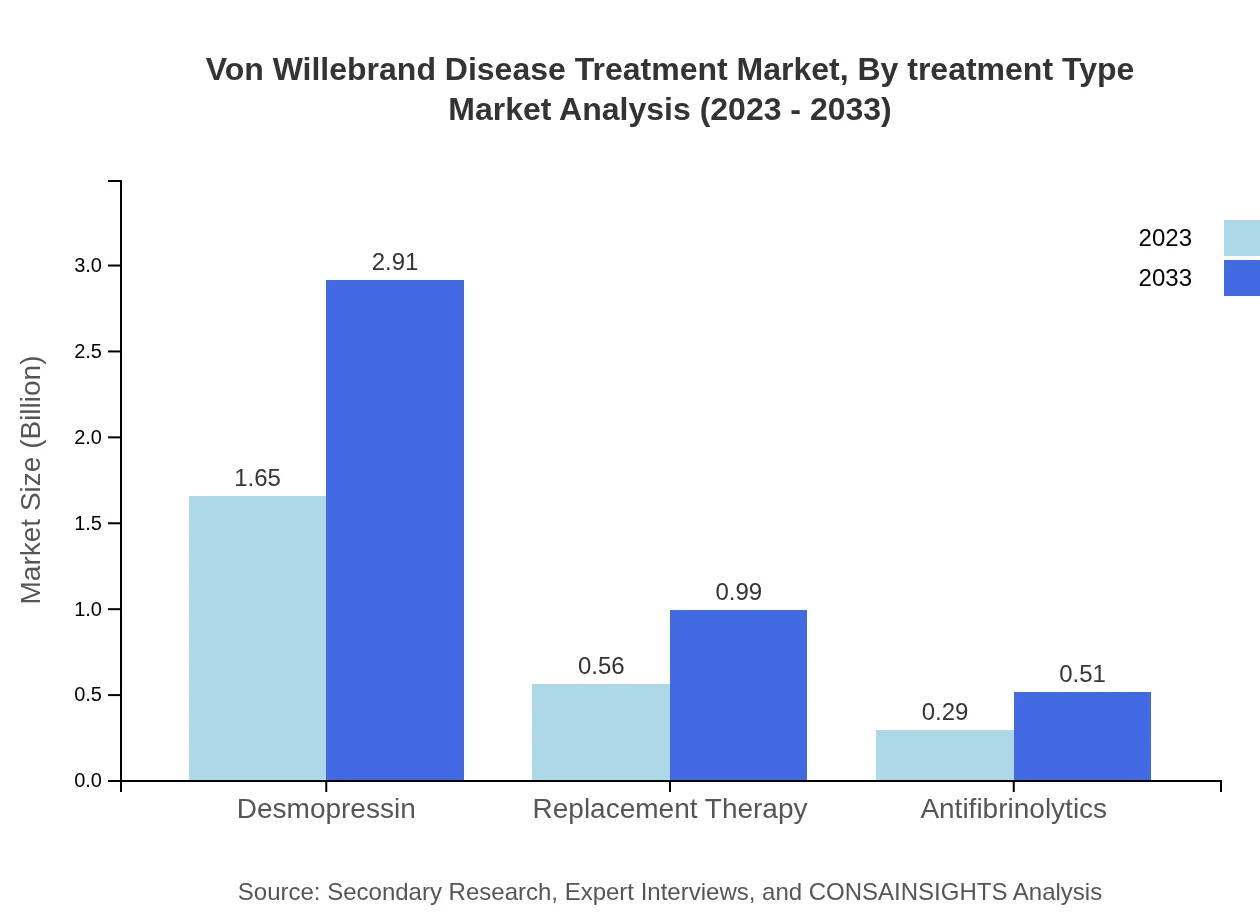
As of 2023, Desmopressin commands the largest portion of the treatment market with a market size of approximately $1.65 billion, representing 66.1% share. The importance of Desmopressin is significant as it serves as the first-line therapy for patients with mild to moderate VWD. Replacement therapy holds a market size of $0.56 billion (22.43% share), highlighting its importance for more severe cases. Antifibrinolytics, prophylactic, and episodic treatments complete the range of therapeutic options available, catering to differing patient needs.
Von Willebrand Disease Treatment Market Analysis By Therapy Strategy
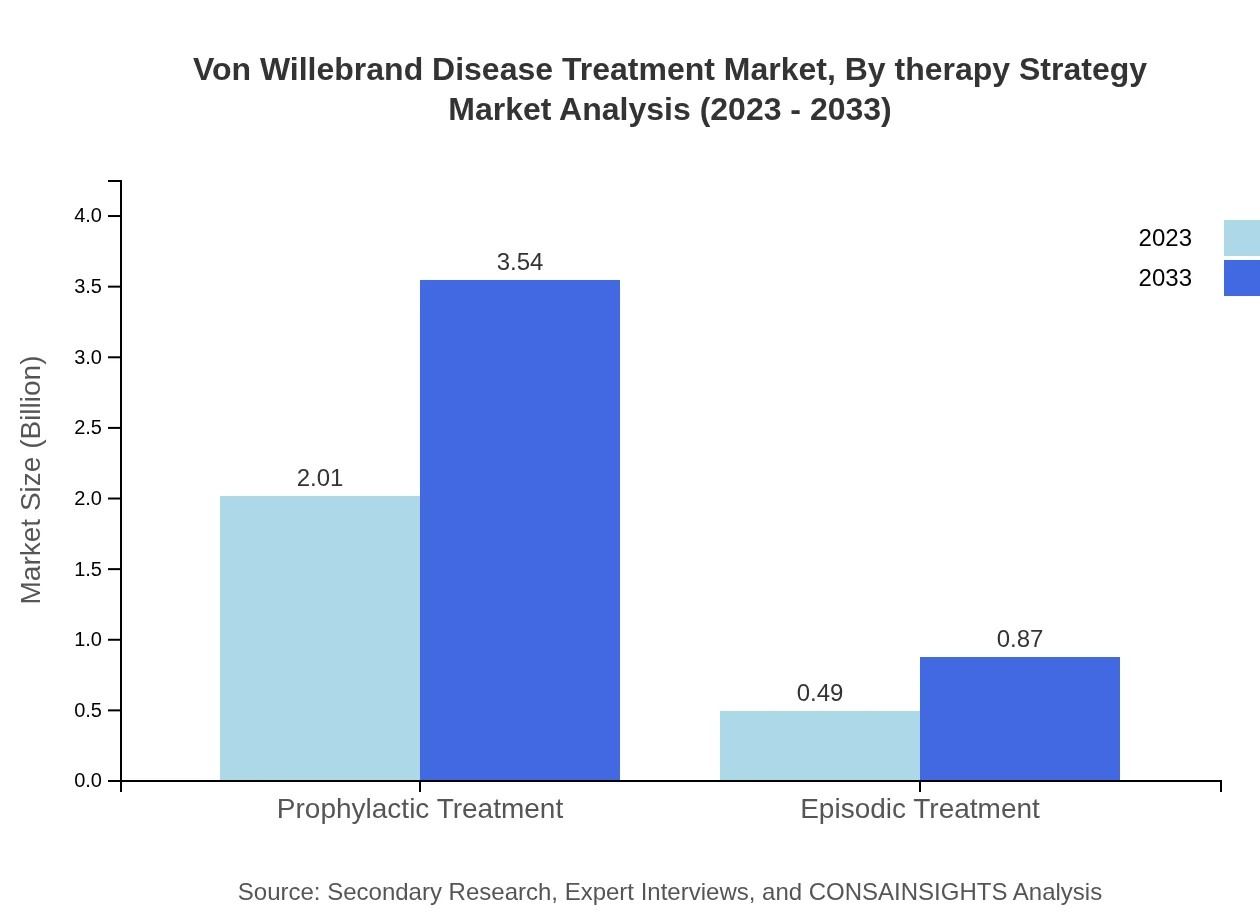
Prophylactic treatment is the most utilized therapy strategy, representing an expansive $2.01 billion market size with an 80.27% share in 2023. This highlights preventive strategies' importance in managing VWD. In contrast, episodic treatments account for $0.49 billion (19.73% share), representing a reactive approach to managing bleeding episodes.
Von Willebrand Disease Treatment Market Analysis By Patient Type
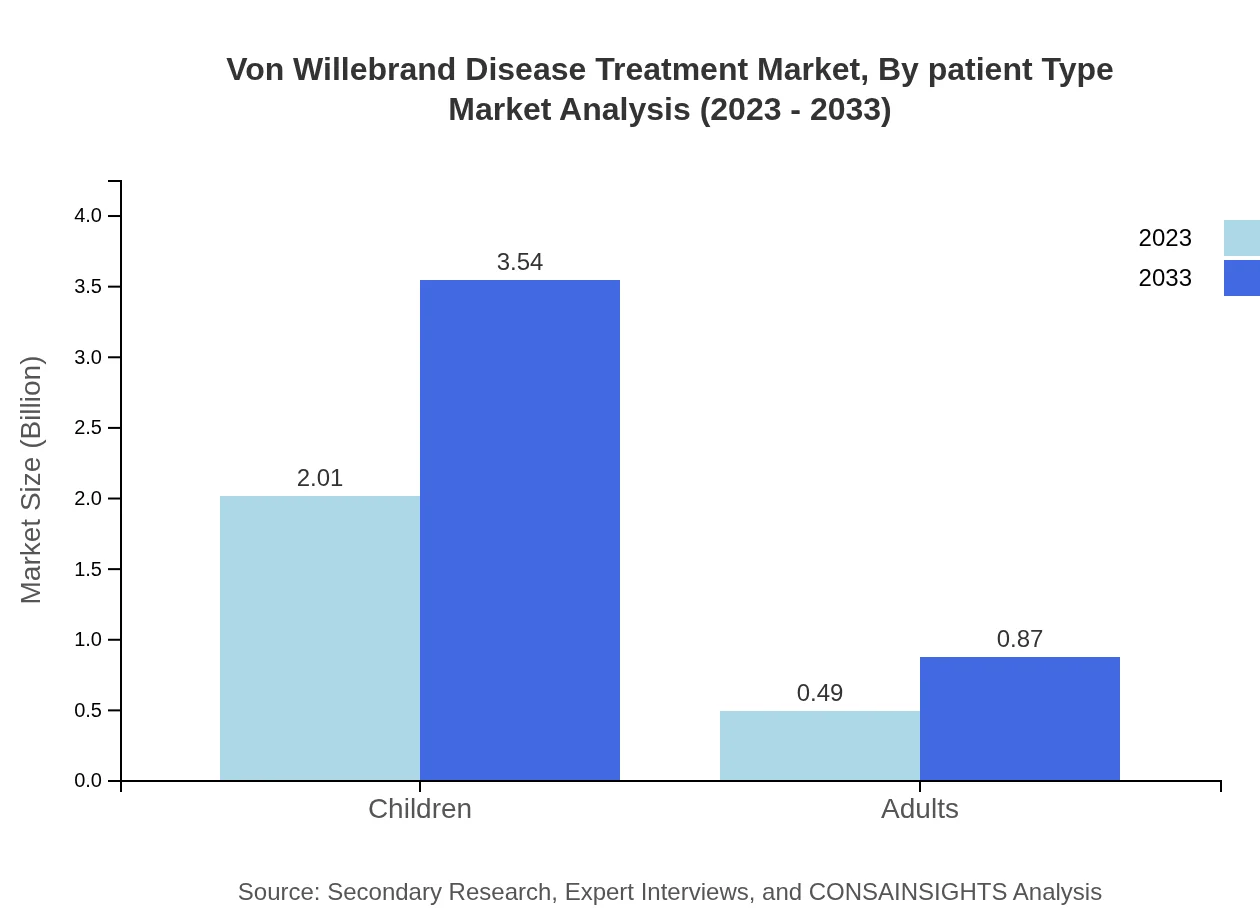
The treatment market for children is projected to rise from $2.01 billion in 2023 to $3.54 billion in 2033, maintaining an 80.27% share, signifying that managing VWD in pediatric populations is a top priority. For adults, the segment is only expected to grow from $0.49 billion with a 19.73% share in 2023, to $0.87 billion in 2033, indicating that tailored approaches for these populations are becoming more prevalent.
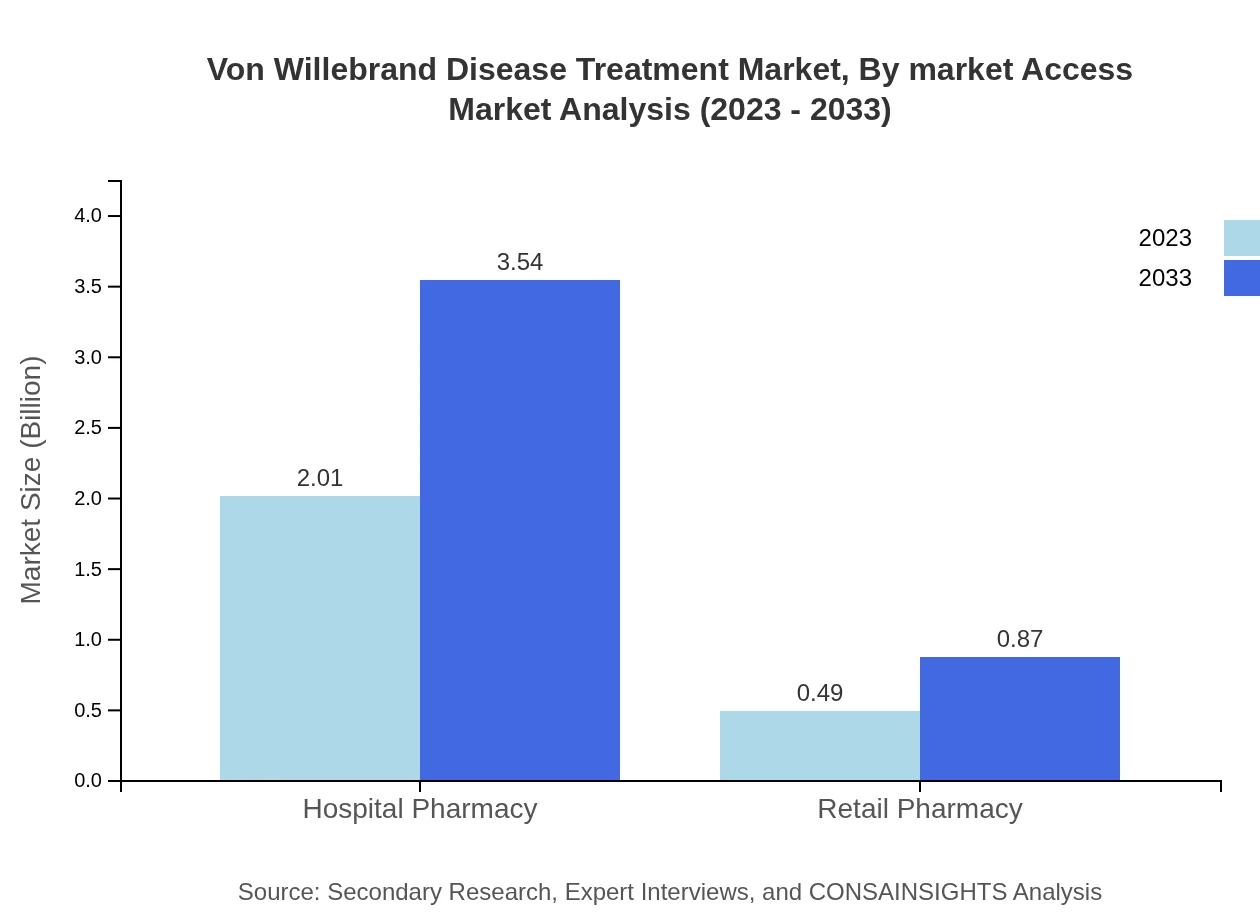
Von Willebrand Disease Treatment Market Analysis By Market Access
The distribution of Von Willebrand Disease treatments through hospital pharmacies is dominating with a market segment valued at $2.01 billion in 2023 and foresighted growth to $3.54 billion by 2033, holding an 80.27% share. Retail pharmacies, while critical, only account for $0.49 billion (19.73% share), reflecting patterns of accessibility and patient choices.
Von Willebrand Disease Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Von Willebrand Disease Treatment Industry
Takeda Pharmaceuticals:
A leading global biopharmaceutical company, Takeda has been instrumental in providing innovative treatments for bleeding disorders, including Von Willebrand Disease.Baxter International Inc.:
Baxter specializes in treatments for hemophilia and has developed various products aimed at treating Von Willebrand Disease, enhancing patient outcomes.CSL Behring:
CSL Behring is known for its commitment to patients with bleeding disorders, providing essential therapies that address various types of VWD.Grifols:
Grifols develops a wide range of plasma-derived therapies and is recognized for its contributions to the treatment of coagulation disorders.We're grateful to work with incredible clients.









FAQs
What is the market size of von Willebrand Disease Treatment?
The global market size for Von Willebrand Disease treatment is currently valued at approximately $2.5 billion. It is projected to grow at a CAGR of 5.7% over the next decade, indicating rising demand for effective therapeutic interventions.
What are the key market players or companies in this von Willebrand Disease Treatment industry?
The key market players in von Willebrand Disease treatment include pharmaceutical companies specializing in hematology, biotechnology firms focused on novel therapeutic approaches, and medical device manufacturers for treatment delivery systems. Collaboration and innovation are critical among these entities.
What are the primary factors driving the growth in the von Willebrand Disease Treatment industry?
Key factors driving growth include increased awareness of von Willebrand Disease, advancements in treatment technologies, rising incidences of bleeding disorders, and a growing focus on personalized medicine, which enhances treatment effectiveness and patient outcomes.
Which region is the fastest Growing in the von Willebrand Disease Treatment?
The Asia Pacific region is the fastest-growing market, expected to expand from $0.52 billion in 2023 to $0.92 billion by 2033. This growth is fueled by improving healthcare infrastructure and increased access to treatment options for patients.
Does ConsaInsights provide customized market report data for the von Willebrand Disease Treatment industry?
Yes, ConsaInsights offers customized market report data for the Von Willebrand Disease treatment sector. Clients can obtain tailored insights addressing specific queries, market dynamics, and growth opportunities according to their needs.
What deliverables can I expect from this von Willebrand Disease Treatment market research project?
Clients can expect comprehensive deliverables including detailed market analyses, segment breakdowns, competitive landscape evaluations, regional insights, and forecasts. Reports will be structured to inform strategic decision-making and identify emerging trends.
What are the market trends of von Willebrand Disease Treatment?
Market trends include an increasing shift towards prophylactic treatment, the development of innovative therapies, growing preferences for home-based treatment options, and advancements in digital health technologies that support patient management and monitoring.