Warfarin Sensitivity Test Market Report
Published Date: 31 January 2026 | Report Code: warfarin-sensitivity-test
Warfarin Sensitivity Test Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Warfarin Sensitivity Test market from 2023 to 2033. It includes insights into market size, growth trends, regional dynamics, and key players, aiming to equip stakeholders with vital information to make informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
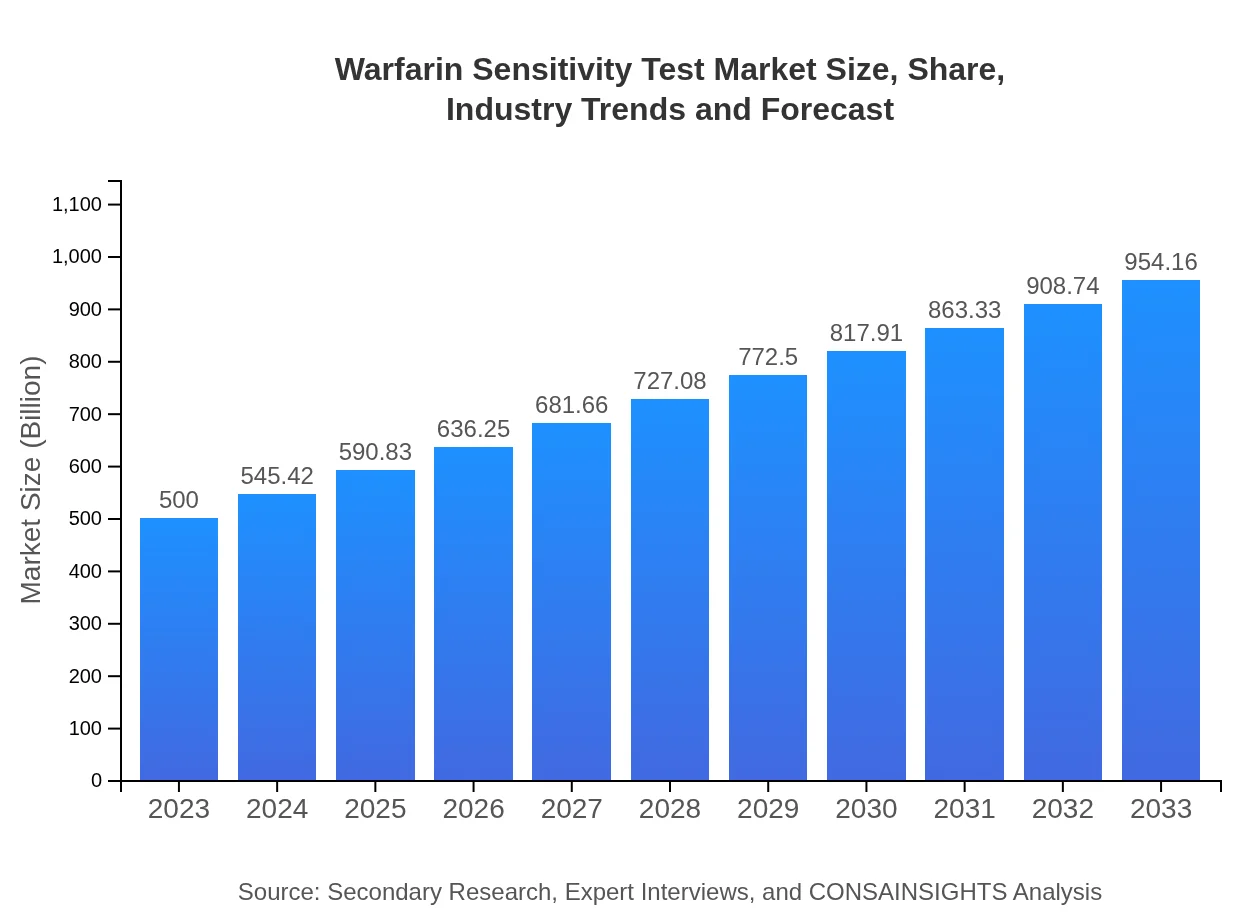
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $954.16 Million |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Genomic Health |
| Last Modified Date | 31 January 2026 |
Warfarin Sensitivity Test Market Overview
Customize Warfarin Sensitivity Test Market Report market research report
- ✔ Get in-depth analysis of Warfarin Sensitivity Test market size, growth, and forecasts.
- ✔ Understand Warfarin Sensitivity Test's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Warfarin Sensitivity Test
What is the Market Size & CAGR of Warfarin Sensitivity Test market in 2023?
Warfarin Sensitivity Test Industry Analysis
Warfarin Sensitivity Test Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Warfarin Sensitivity Test Market Analysis Report by Region
Europe Warfarin Sensitivity Test Market Report:
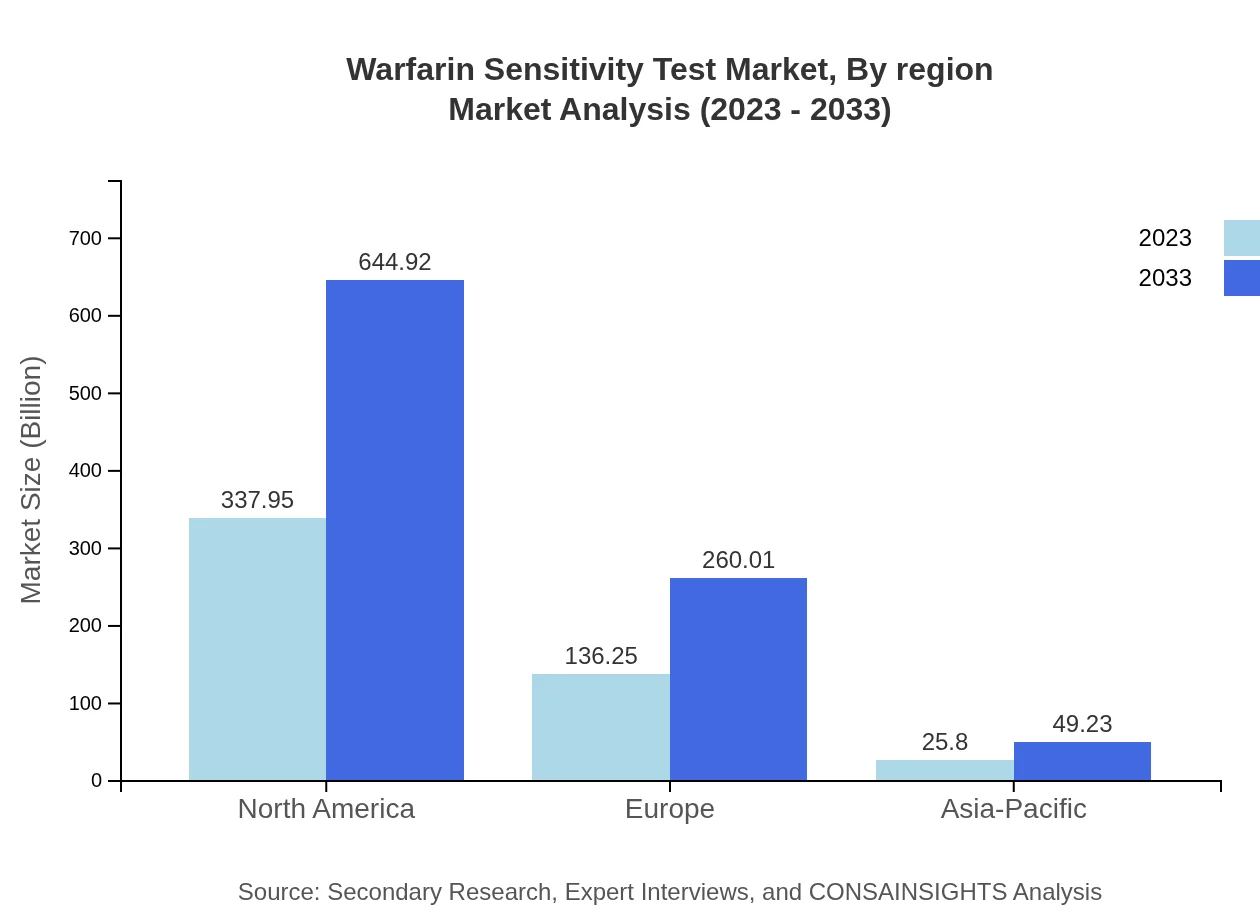
In Europe, the market size is projected to grow from $180.95 million in 2023 to $345.31 million by 2033. The European market is characterized by regulatory support for pharmacogenetic testing, which aids in fair reimbursement strategies for these services.Asia Pacific Warfarin Sensitivity Test Market Report:
In the Asia Pacific region, the Warfarin Sensitivity Test market is projected to grow from $77.50 million in 2023 to $147.89 million by 2033, driven by increasing investments in healthcare infrastructure and rising incidences of cardiovascular diseases. The region is witnessing growth in genetic testing capabilities, enhancing the availability and adoption of warfarin sensitivity tests.North America Warfarin Sensitivity Test Market Report:
North America is the largest market for Warfarin Sensitivity Tests, with an expected increase from $167.85 million in 2023 to $320.31 million by 2033, primarily due to a high adoption rate of genetic testing in clinical settings and advanced healthcare facilities.South America Warfarin Sensitivity Test Market Report:
The South America region's market is expected to expand from $44.90 million in 2023 to $85.68 million by 2033. Factors such as an increasing elderly population and the rising prevalence of thromboembolic disorders drive demand for more accurate testing solutions.Middle East & Africa Warfarin Sensitivity Test Market Report:
The Middle East and Africa market is initially smaller, expanding from $28.80 million in 2023 to $54.96 million by 2033, driven largely by improving healthcare infrastructure and increasing awareness surrounding personalized medicine.Tell us your focus area and get a customized research report.
Warfarin Sensitivity Test Market Analysis By Test Type
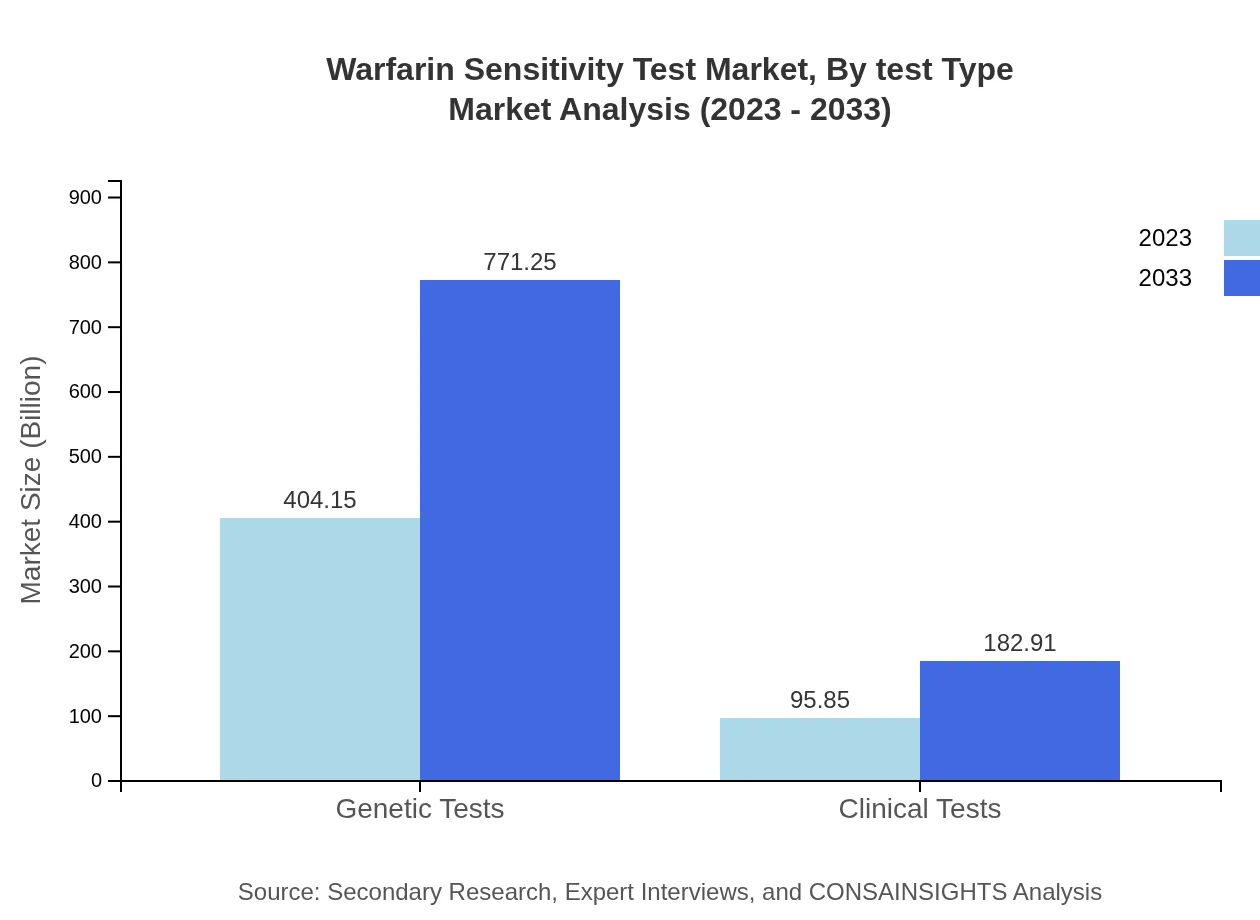
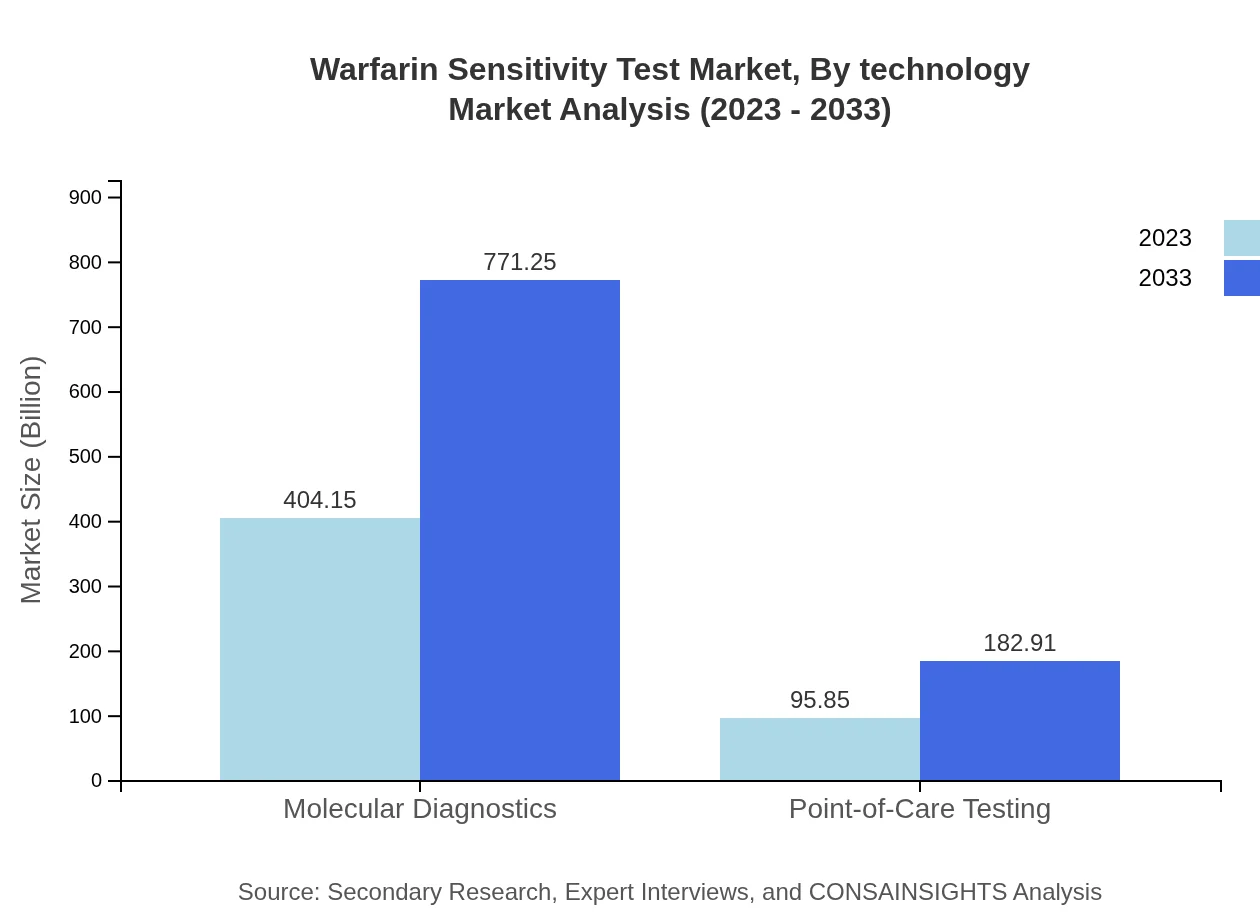
The Warfarin Sensitivity Test, by test type, includes Genetic Tests ($404.15 million in 2023), Clinical Tests ($95.85 million in 2023), Molecular Diagnostics ($404.15 million in 2023), Point-of-Care Testing ($95.85 million in 2023), and Home Testing ($25.80 million in 2023). Genetic Tests dominate the market due to their precision in predicting warfarin sensitivity, while Point-of-Care Testing is rising due to its convenience.
Warfarin Sensitivity Test Market Analysis By End User
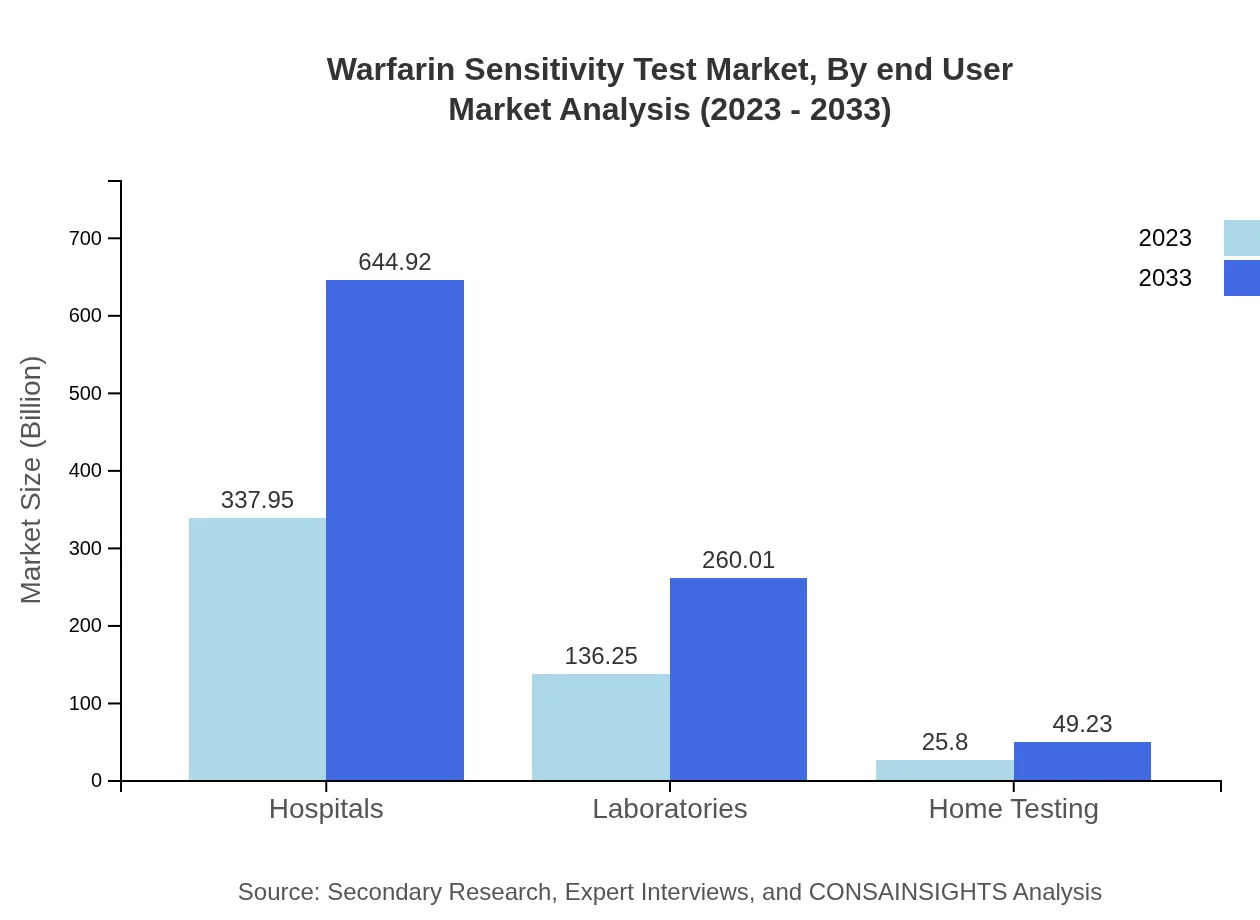
End-user segmentation includes Hospitals (largely driving market size with $337.95 million in 2023), Laboratories ($136.25 million in 2023), and Home Testing ($25.80 million in 2023). Hospitals remain the primary consumers due to their high patient volume, while laboratories are increasingly developing partnerships to improve access to testing technologies.
Warfarin Sensitivity Test Market Analysis By Region
Market analysis by region showcases significant variances: North America is the largest, followed by Europe, with Asia-Pacific on a growth trajectory due to heightened healthcare investment. Each region's specific regulatory environments and healthcare demands shape the unique market landscape.
Warfarin Sensitivity Test Market Analysis By Technology
Technological advancements including high-throughput sequencing and innovative point-of-care devices are crucial in the Warfarin Sensitivity Test market. These technologies facilitate faster, more accurate testing and assist in real-time decision-making for treatment protocols.
Warfarin Sensitivity Test Market Analysis By Distribution Channel
Distribution channels encompass online and offline sales, with online sales gaining traction due to increasing preference for convenience and accessibility. The segment is projected to lead in growth due to the ongoing digitization of healthcare services, especially during the post-pandemic recovery phase.
Warfarin Sensitivity Test Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Warfarin Sensitivity Test Industry
Roche Diagnostics:
Roche Diagnostics is a leading global healthcare company specializing in diagnostic solutions. Their advanced genetic testing capabilities have greatly contributed to the accuracy of Warfarin Sensitivity Tests.Abbott Laboratories:
Abbott Laboratories is known for its innovative healthcare products, emphasizing personalized medicine. Their contributions to point-of-care testing technologies have enhanced accessibility to warfarin sensitivity testing.Siemens Healthineers:
Siemens Healthineers provides cutting-edge diagnostic technologies and solutions. Their focus on molecular diagnostics plays a critical role in personalized anticoagulant therapy.Genomic Health:
As a pioneer in the field of genomic diagnostics, Genomic Health provides reliable and precise genetic tests which are integral to evaluating warfarin sensitivity.We're grateful to work with incredible clients.









FAQs
What is the market size of Warfarin Sensitivity Test?
The global market size for the Warfarin Sensitivity Test was estimated at approximately $500 million in 2023. It is projected to grow at a CAGR of 6.5%, indicating significant growth potential over the next decade.
What are the key market players or companies in the Warfarin Sensitivity Test industry?
The Warfarin Sensitivity Test market features key players such as Roche, Siemens Healthineers, Abbott Laboratories, and Thermo Fisher Scientific. These companies are instrumental in developing innovative testing solutions and expanding their market presence.
What are the primary factors driving the growth in the Warfarin Sensitivity Test industry?
Key growth factors include the increasing prevalence of cardiovascular diseases, rising awareness of personalized medicine, advancements in molecular diagnostics, and the growing need for precision in anticoagulation therapy.
Which region is the fastest Growing in the Warfarin Sensitivity Test?
The Asia-Pacific region exhibits rapid growth in the Warfarin Sensitivity Test market, projected to increase from $77.50 million in 2023 to $147.89 million by 2033, fueled by rising healthcare investments and increasing disease prevalence.
Does ConsaInsights provide customized market report data for the Warfarin Sensitivity Test industry?
Yes, ConsaInsights offers tailored market reports for the Warfarin Sensitivity Test industry, accommodating specific client needs and providing detailed insights on market trends, competitive analysis, and growth opportunities.
What deliverables can I expect from this Warfarin Sensitivity Test market research project?
Deliverables typically include in-depth market analysis, segmentation data, regional insights, competitive landscape, and forecasts. Customized reports can focus on specific areas like pricing analysis, market dynamics, and strategic recommendations.
What are the market trends of Warfarin Sensitivity Test?
Current trends in the Warfarin Sensitivity Test market include increasing adoption of genetic testing, the shift towards home-based testing solutions, and advancements in point-of-care testing technology, enhancing patient convenience and engagement.