Zika Virus Vaccines Market Report
Published Date: 31 January 2026 | Report Code: zika-virus-vaccines
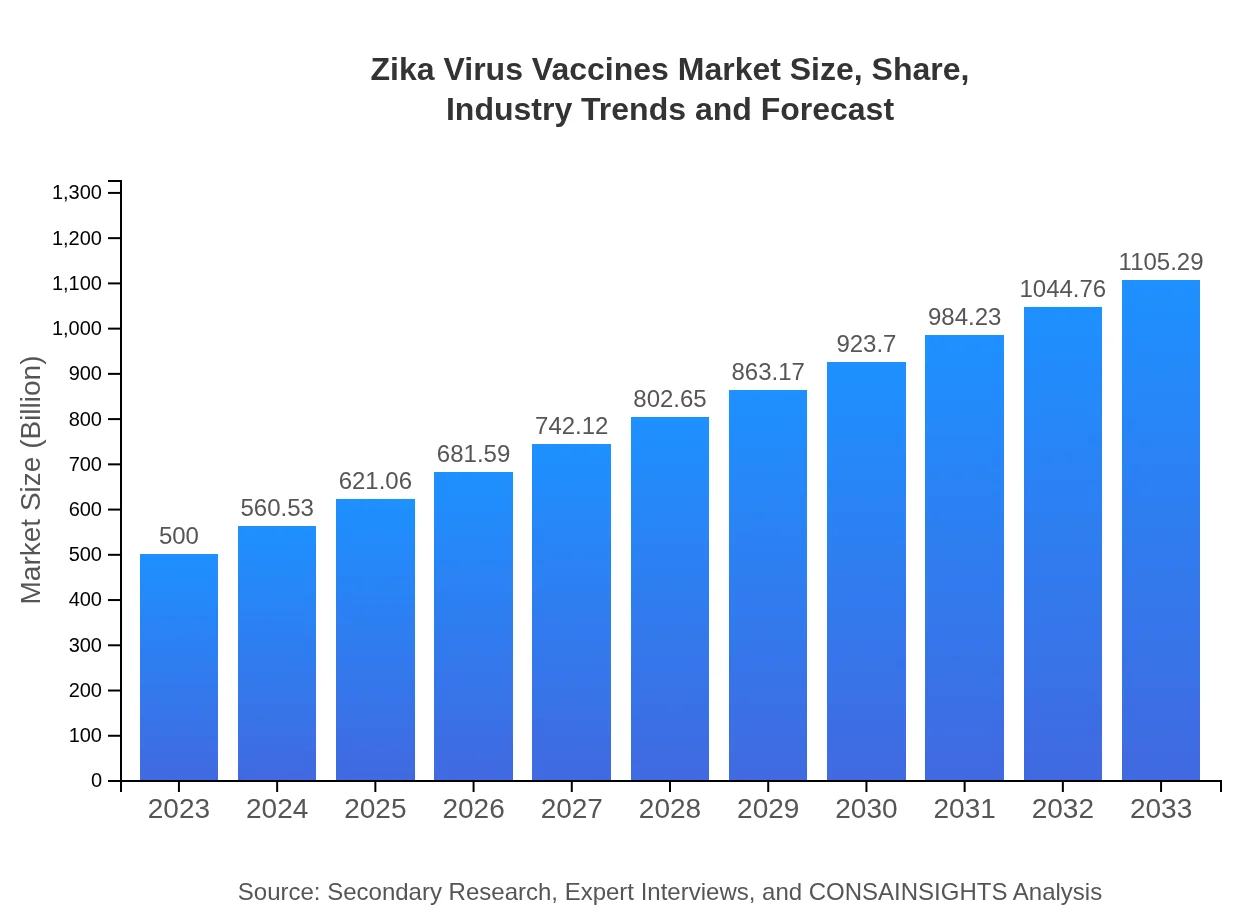
Zika Virus Vaccines Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Zika Virus Vaccines market, emphasizing market size, growth drivers, and forecasts from 2023 to 2033, along with insights into trends, regional performances, and key segments within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 8% |
| 2033 Market Size | $1105.29 Million |
| Top Companies | Sanofi Pasteur, GlaxoSmithKline (GSK), Inovio Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Zika Virus Vaccines Market Overview
Customize Zika Virus Vaccines Market Report market research report
- ✔ Get in-depth analysis of Zika Virus Vaccines market size, growth, and forecasts.
- ✔ Understand Zika Virus Vaccines's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Zika Virus Vaccines
What is the Market Size & CAGR of Zika Virus Vaccines market in 2023?
Zika Virus Vaccines Industry Analysis
Zika Virus Vaccines Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Zika Virus Vaccines Market Analysis Report by Region
Europe Zika Virus Vaccines Market Report:
The European market is anticipated to rise from $161.10 million in 2023 to $356.12 million by 2033. Growing collaboration among countries to combat infectious diseases will further enhance the market potential.Asia Pacific Zika Virus Vaccines Market Report:
In the Asia Pacific region, the Zika Virus Vaccines market is projected to grow from $96.05 million in 2023 to $212.33 million by 2033. This growth is driven by increased government initiatives in health infrastructure and rising public health awareness regarding Zika virus prevention.North America Zika Virus Vaccines Market Report:
North America leads the Zika Virus Vaccines market with an estimated growth from $165.30 million in 2023 to $365.41 million by 2033. High R&D investment, strong regulatory support, and the presence of biotechnology firms significantly bolster market dynamics.South America Zika Virus Vaccines Market Report:
The South American market is expected to expand from $32.00 million in 2023 to $70.74 million by 2033, largely because of historical outbreaks and continuing public health campaigns emphasizing vaccination.Middle East & Africa Zika Virus Vaccines Market Report:
In the Middle East and Africa, the market is projected to grow from $45.55 million in 2023 to $100.69 million by 2033. Increasing awareness about Zika and its implications on public health is fostering investment in vaccine development.Tell us your focus area and get a customized research report.
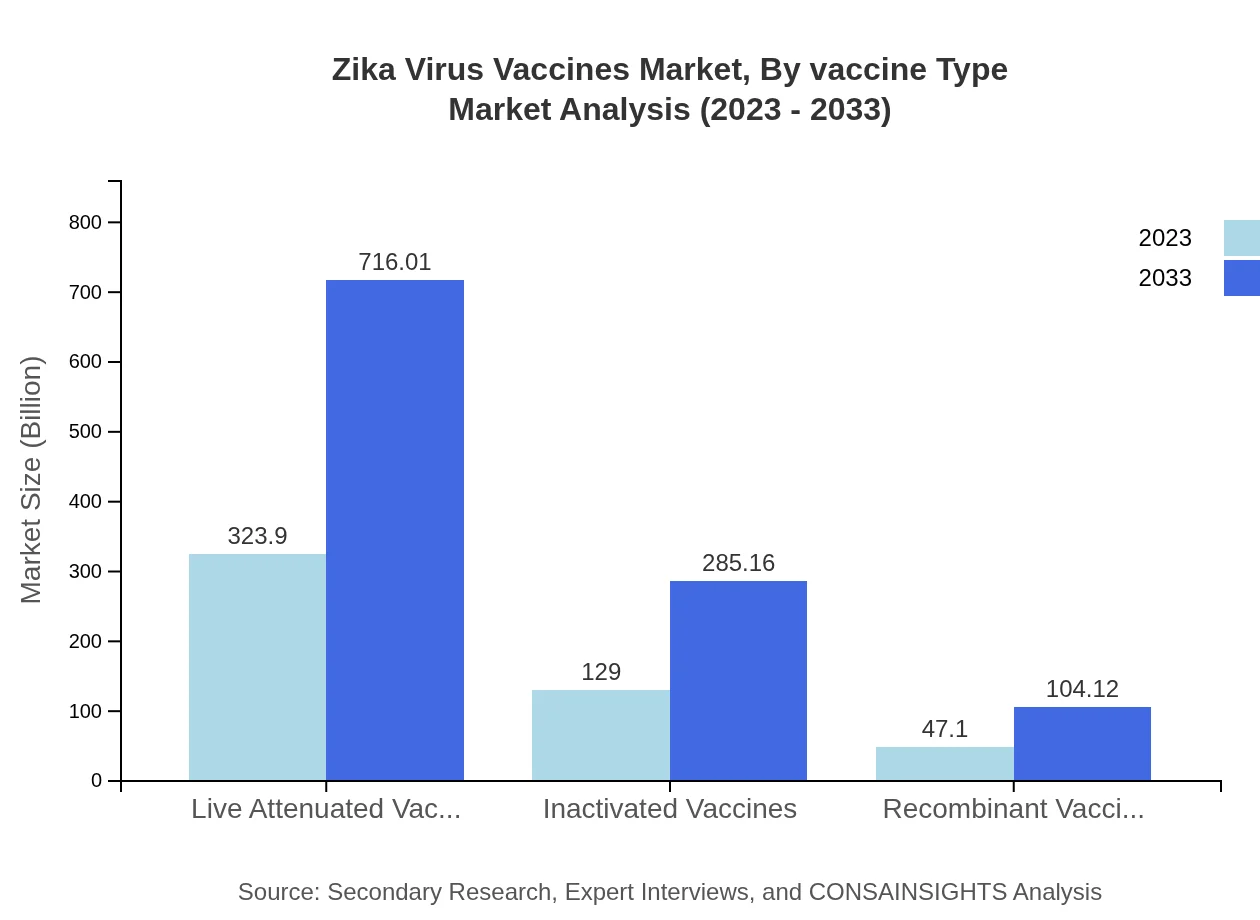
Zika Virus Vaccines Market Analysis By Vaccine Type
The Zika Virus Vaccines market can be categorized into three main vaccine types: live attenuated, inactivated, and recombinant vaccines. As of 2023, live attenuated vaccines dominate with a market value of $323.90 million, translating to a 64.78% market share, while inactivated vaccines account for $129.00 million (25.8% share). Recombinant vaccines hold a smaller segment at $47.10 million (9.42% share). The anticipated growth in these areas reflects advancements in technology and a greater emphasis on safety.
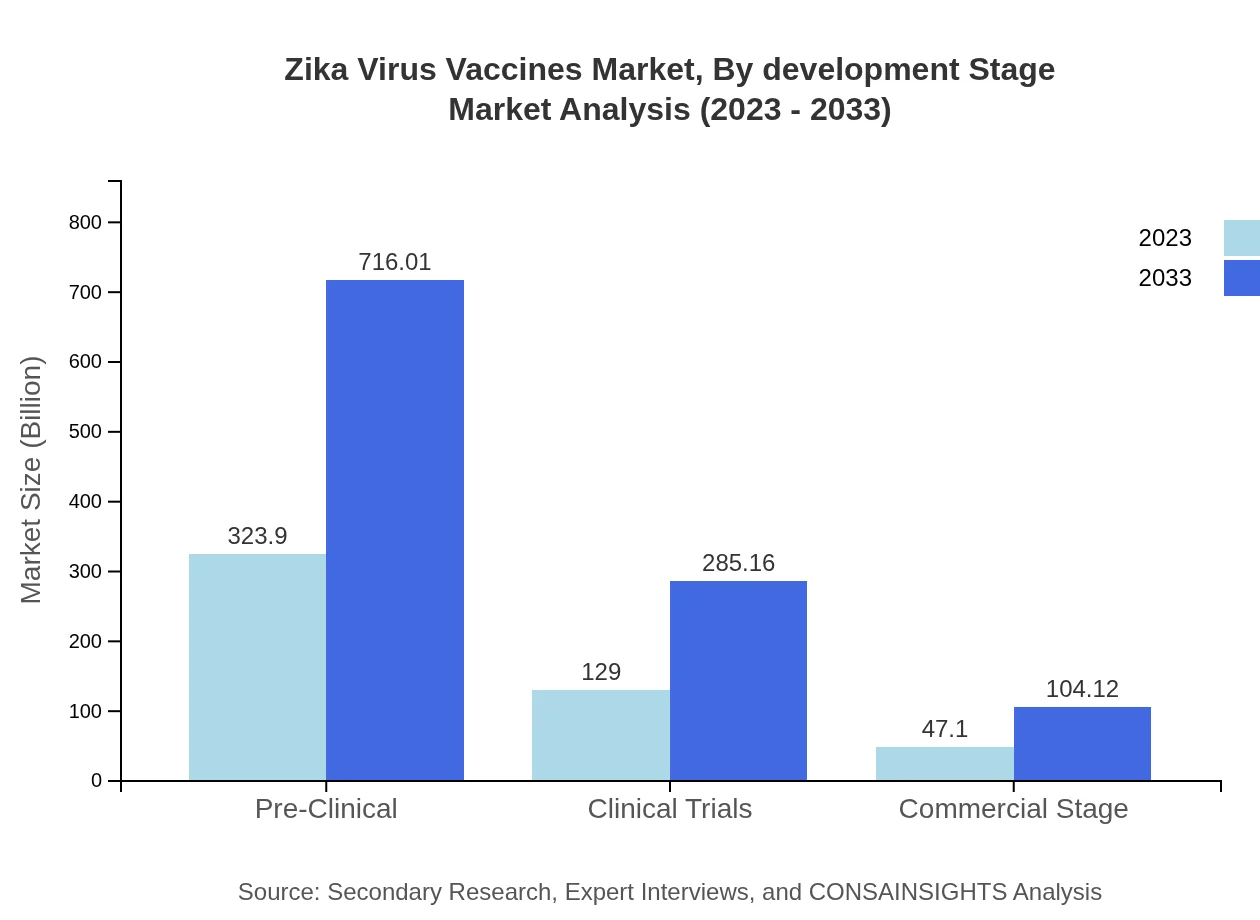
Zika Virus Vaccines Market Analysis By Development Stage
Segmentation by the development stage includes pre-clinical, clinical trials, and commercial stage vaccines. Pre-clinical vaccines lead the scope, valued at $323.90 million in 2023 (64.78% share), followed by products in clinical trials at $129.00 million (25.8% share) and commercial stage products at $47.10 million (9.42% share). Increased investment in R&D during the pre-clinical phases fuels market stability.
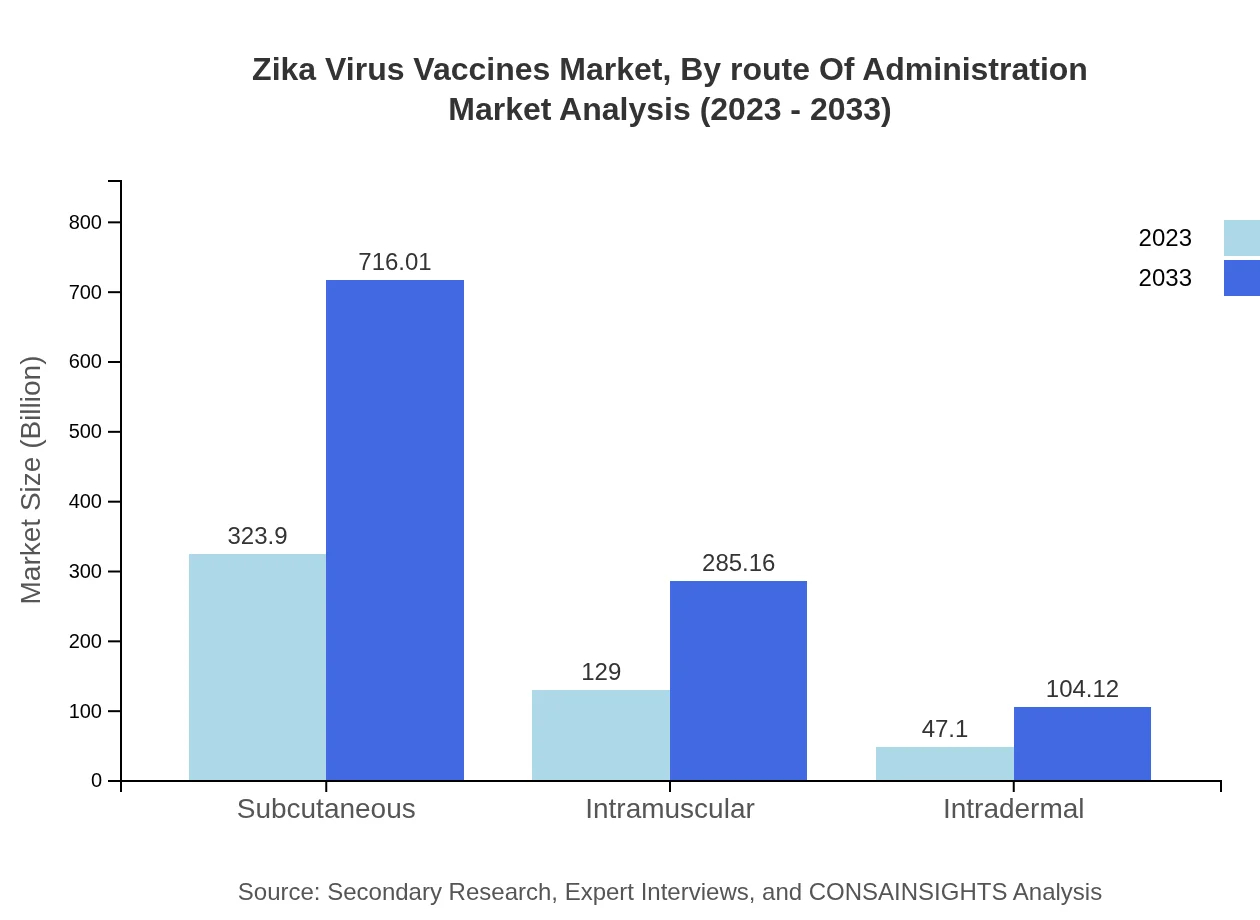
Zika Virus Vaccines Market Analysis By Route Of Administration
Vaccines are also classified based on the route of administration: subcutaneous, intramuscular, and intradermal. Subcutaneous vaccines are the most prevalent, commanding a market worth $323.90 million (64.78% share) in 2023, while intramuscular vaccines follow closely with $129.00 million (25.8% share) and intradermal vaccines at $47.10 million (9.42% share). The choice of administration route is critical for optimal patient acceptability and vaccine uptake.
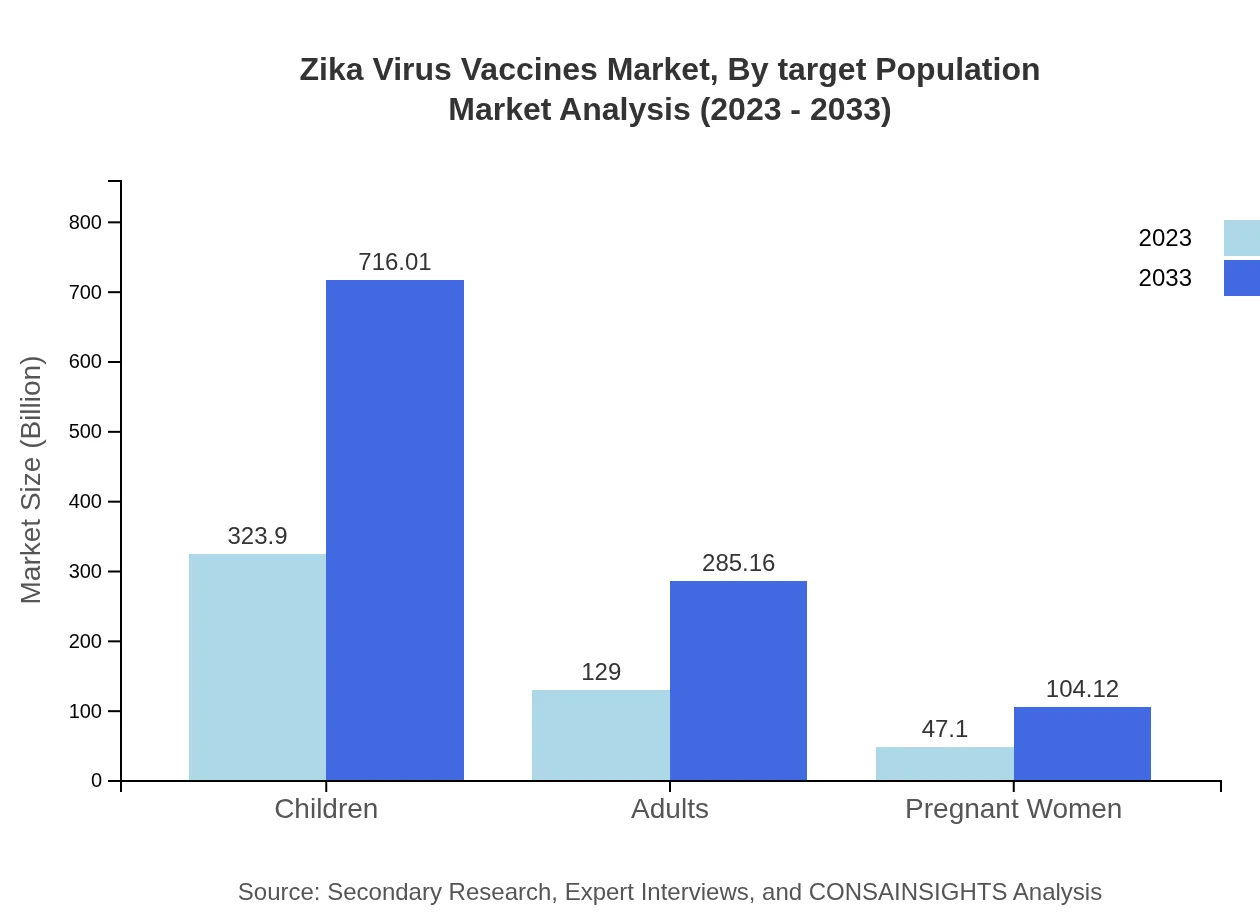
Zika Virus Vaccines Market Analysis By Target Population
Target populations for Zika vaccines include children, adults, and pregnant women. Children represent the largest segment, with a market size of $323.90 million (64.78% share). Adults hold $129.00 million (25.8% share), while pregnant women constitute a smaller but growing market segment at $47.10 million (9.42% share). Tailored vaccine formulations for specific demographics enhance health outcomes and preventive care.
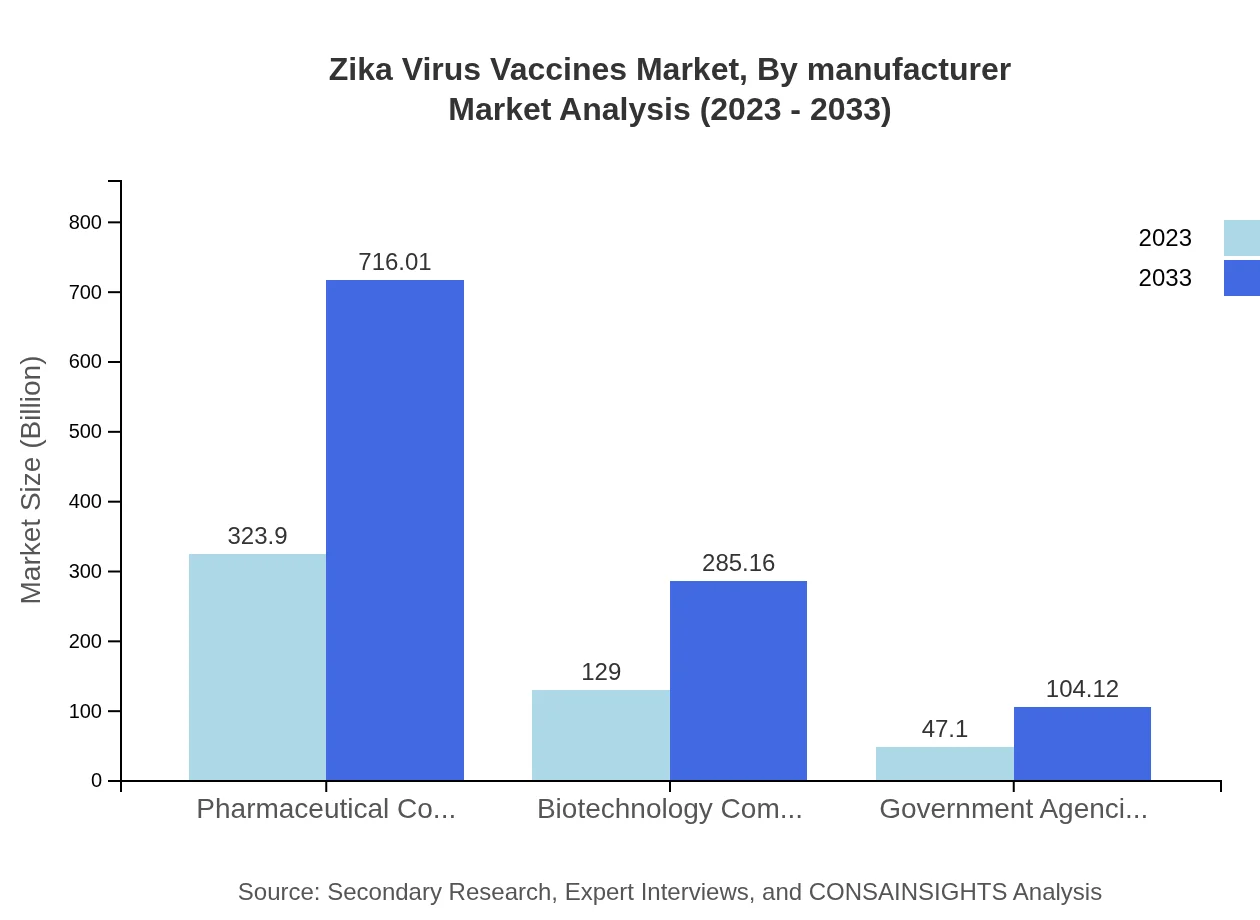
Zika Virus Vaccines Market Analysis By Manufacturer
Manufacturers in the Zika Virus Vaccines market comprise pharmaceutical and biotechnology companies. Analysis shows that large pharmaceutical companies dominate the industry, contributing to $323.90 million (64.78% share) in market size, while biotechnology firms are increasingly becoming players with $129.00 million (25.8% share). Government agencies play a supporting role with $47.10 million (9.42% share), particularly in funding and conducting clinical trials.
Zika Virus Vaccines Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Zika Virus Vaccines Industry
Sanofi Pasteur:
A leading global healthcare company, Sanofi Pasteur is notable for its pioneering work in developing Zika virus vaccines afte outbreaks in various countries. Their efforts aim to enhance immunization coverage in endemic regions.GlaxoSmithKline (GSK):
With a strong emphasis on research and development, GSK is committed to advancing vaccine technology and has been actively involved in Zika virus vaccine trials, focusing on public health initiatives and prevention strategies.Inovio Pharmaceuticals:
Inovio specializes in DNA-based vaccines. Their innovative approach to Zika vaccines particularly addresses the urgent need for effective preventive measures in endemic areas, contributing significant research advancements to the field.We're grateful to work with incredible clients.