Pandemic Influenza Vaccine Market Report
Published Date: 31 January 2026 | Report Code: pandemic-influenza-vaccine
Pandemic Influenza Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pandemic Influenza Vaccine market, complete with insights on segmentation, regional performance, growth forecasts, and key industry trends from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
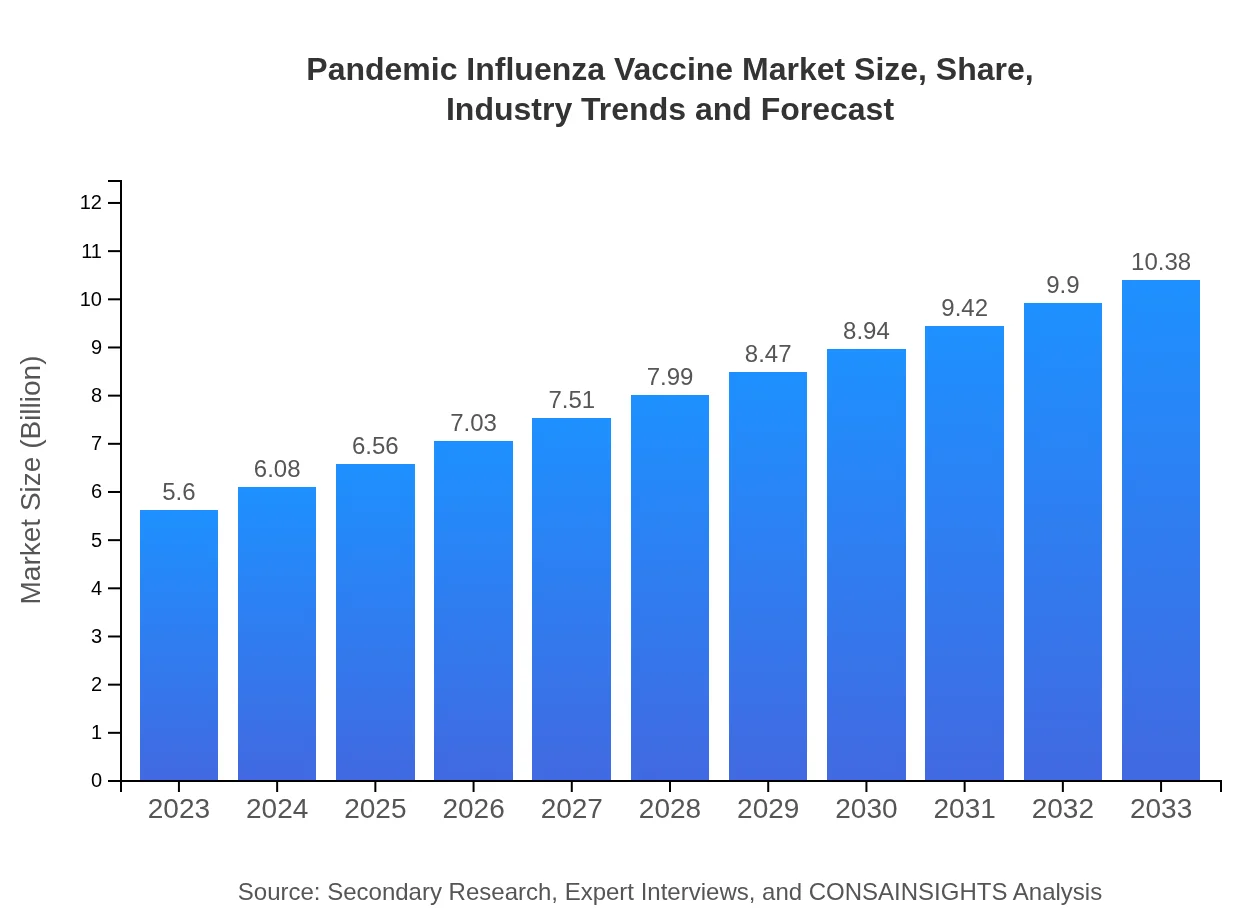
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $10.38 Billion |
| Top Companies | Pfizer Inc., Sanofi Pasteur, GlaxoSmithKline (GSK), Moderna, Inc., Merck & Co. |
| Last Modified Date | 31 January 2026 |
Pandemic Influenza Vaccine Market Overview
Customize Pandemic Influenza Vaccine Market Report market research report
- ✔ Get in-depth analysis of Pandemic Influenza Vaccine market size, growth, and forecasts.
- ✔ Understand Pandemic Influenza Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pandemic Influenza Vaccine
What is the Market Size & CAGR of Pandemic Influenza Vaccine market in 2023?
Pandemic Influenza Vaccine Industry Analysis
Pandemic Influenza Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pandemic Influenza Vaccine Market Analysis Report by Region
Europe Pandemic Influenza Vaccine Market Report:
The European market is projected to rise from $1.50 billion in 2023 to $2.79 billion in 2033. Strict regulatory frameworks and a high rate of vaccination acceptance among the population contribute to the region's market dynamics.Asia Pacific Pandemic Influenza Vaccine Market Report:
In the Asia Pacific region, the market is anticipated to grow from $1.13 billion in 2023 to $2.09 billion by 2033. Growing populations and increased government focus on vaccine development post-pandemic have significantly boosted market potential in countries such as India and China.North America Pandemic Influenza Vaccine Market Report:
North America is a major market, with estimates of $2.02 billion in 2023, growing to $3.75 billion by 2033. The presence of key players, robust healthcare systems, and increased public health initiatives underpin this growth.South America Pandemic Influenza Vaccine Market Report:
The South American market is expected to increase from $0.18 billion in 2023 to $0.33 billion in 2033, driven by improving healthcare infrastructure and the emphasis on vaccination against influenza outbreaks.Middle East & Africa Pandemic Influenza Vaccine Market Report:
The Middle East and Africa market is expected to grow from $0.77 billion in 2023 to $1.43 billion by 2033, supported by increasing funding for healthcare initiatives and awareness campaigns regarding the importance of vaccinations.Tell us your focus area and get a customized research report.
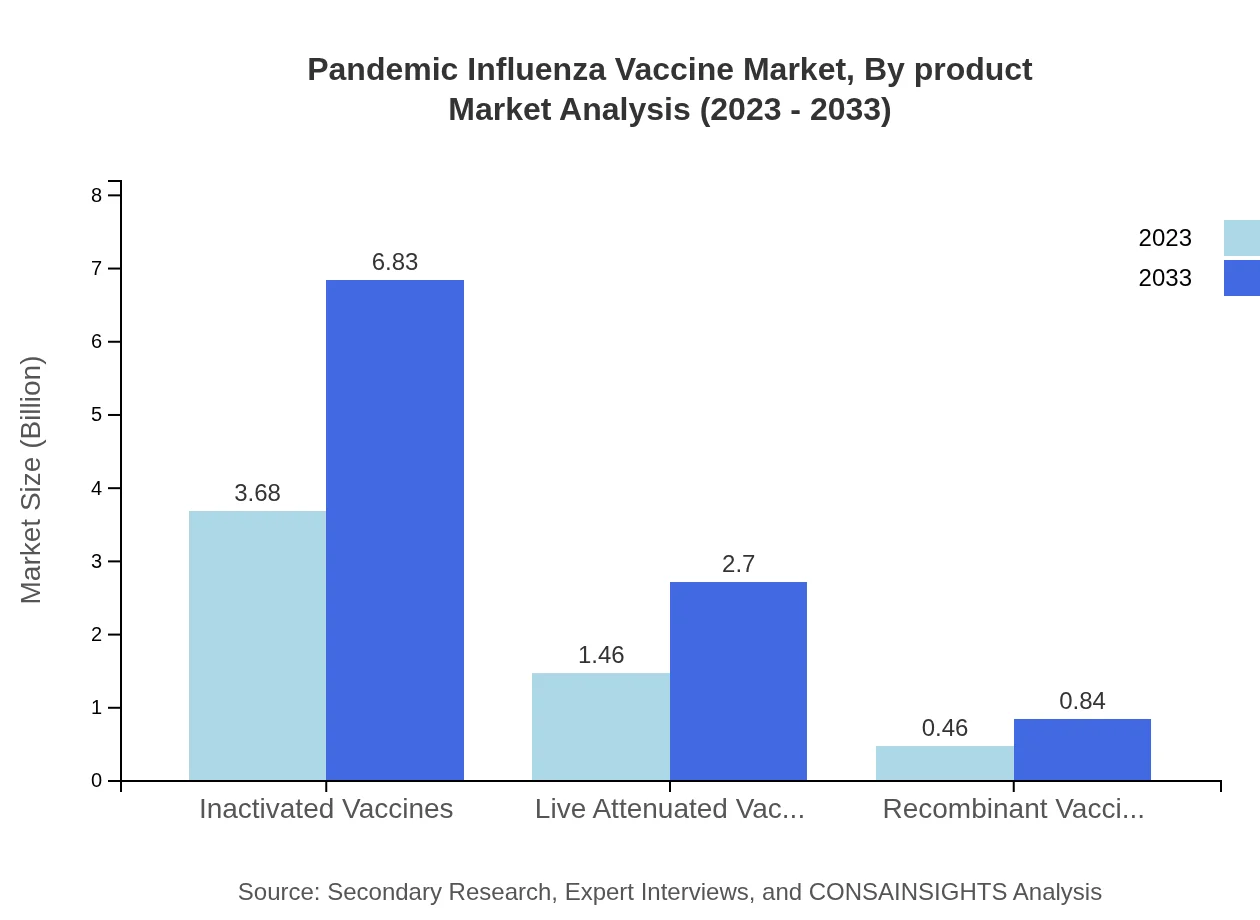
Pandemic Influenza Vaccine Market Analysis By Product
The analysis shows that Inactivated Vaccines dominate the market, representing a size of $3.68 billion in 2023, expected to grow to $6.83 billion by 2033, maintaining a market share of 65.79%. Live Attenuated Vaccines accounted for $1.46 billion in 2023, with projections of $2.70 billion by 2033 and a consistent market share of 26.07%. Recombinant Vaccines start smaller with $0.46 billion, expected to reach $0.84 billion.
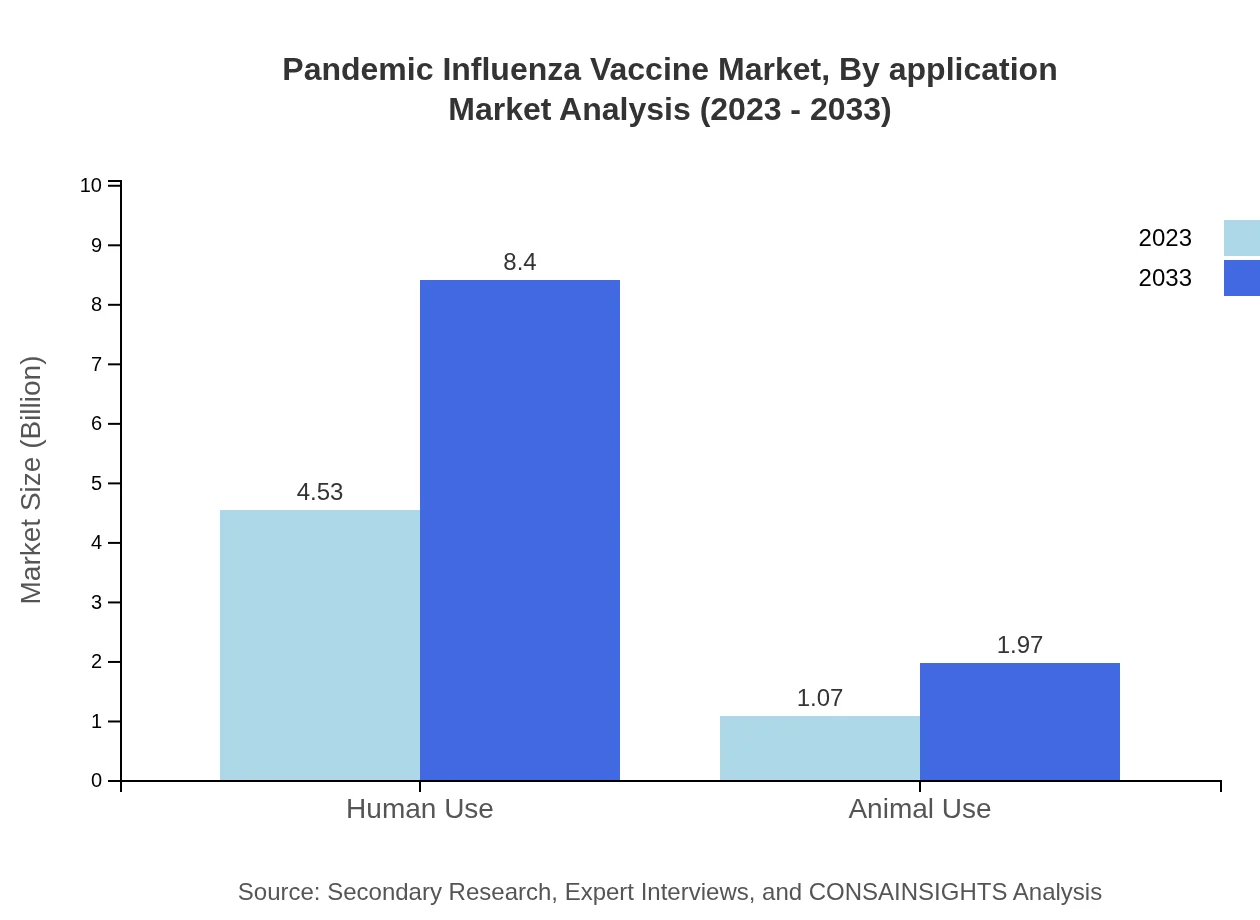
Pandemic Influenza Vaccine Market Analysis By Application
When analyzed by application, Human Use comprises a significant $4.53 billion in 2023, projected to increase to $8.40 billion in 2033, holding a market share of 80.98%. Conversely, the Animal Use segment at $1.07 billion is forecasted to grow to $1.97 billion, maintaining a market share of 19.02%. This segmentation highlights the larger societal focus on human health concerning pandemic influenza.
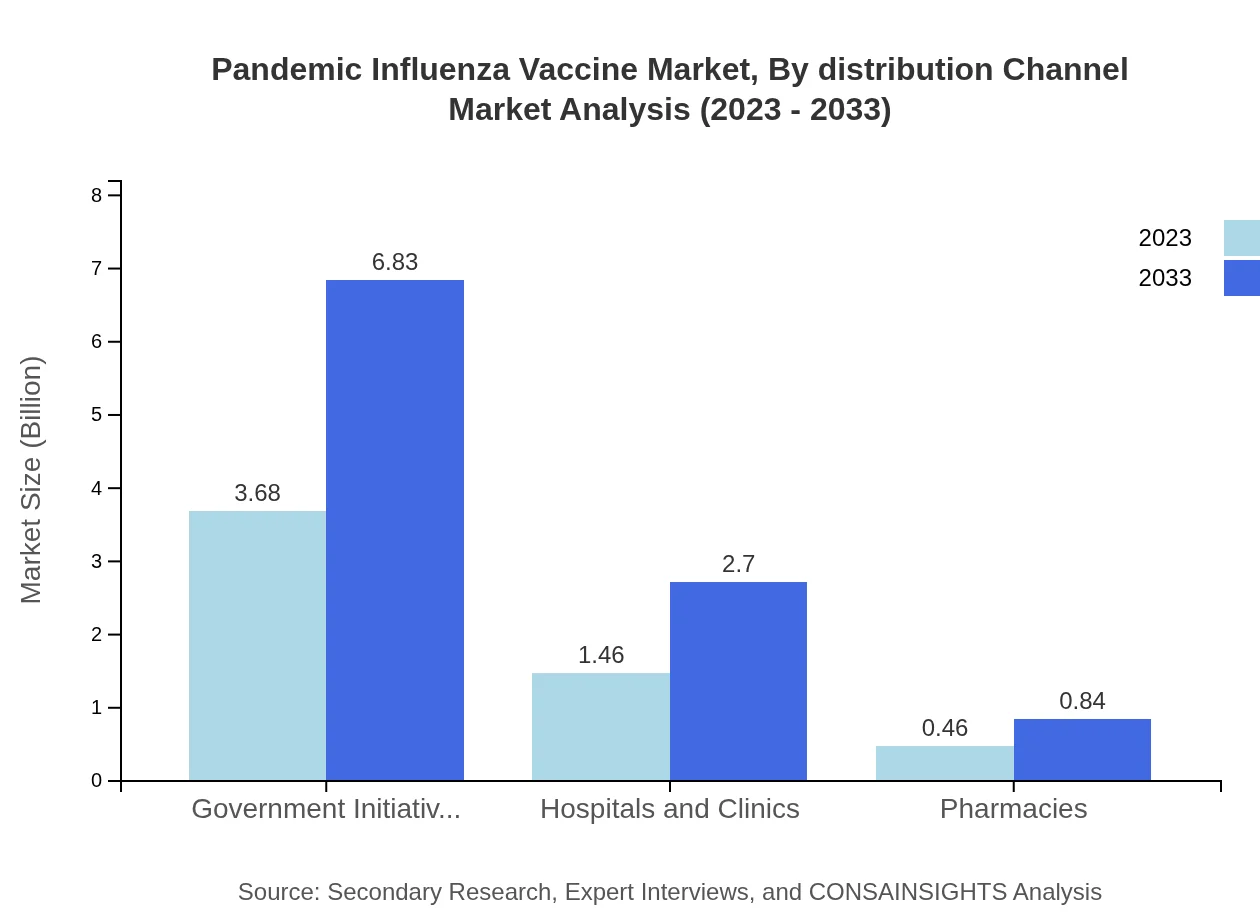
Pandemic Influenza Vaccine Market Analysis By Distribution Channel
Distribution analysis reveals that Government Initiatives dominate with $3.68 billion in 2023, expected to increase to $6.83 billion by the end of the forecast period, reaffirming their 65.79% market share. Hospitals and Clinics follow with $1.46 billion, while Pharmacies represent a niche at $0.46 billion.
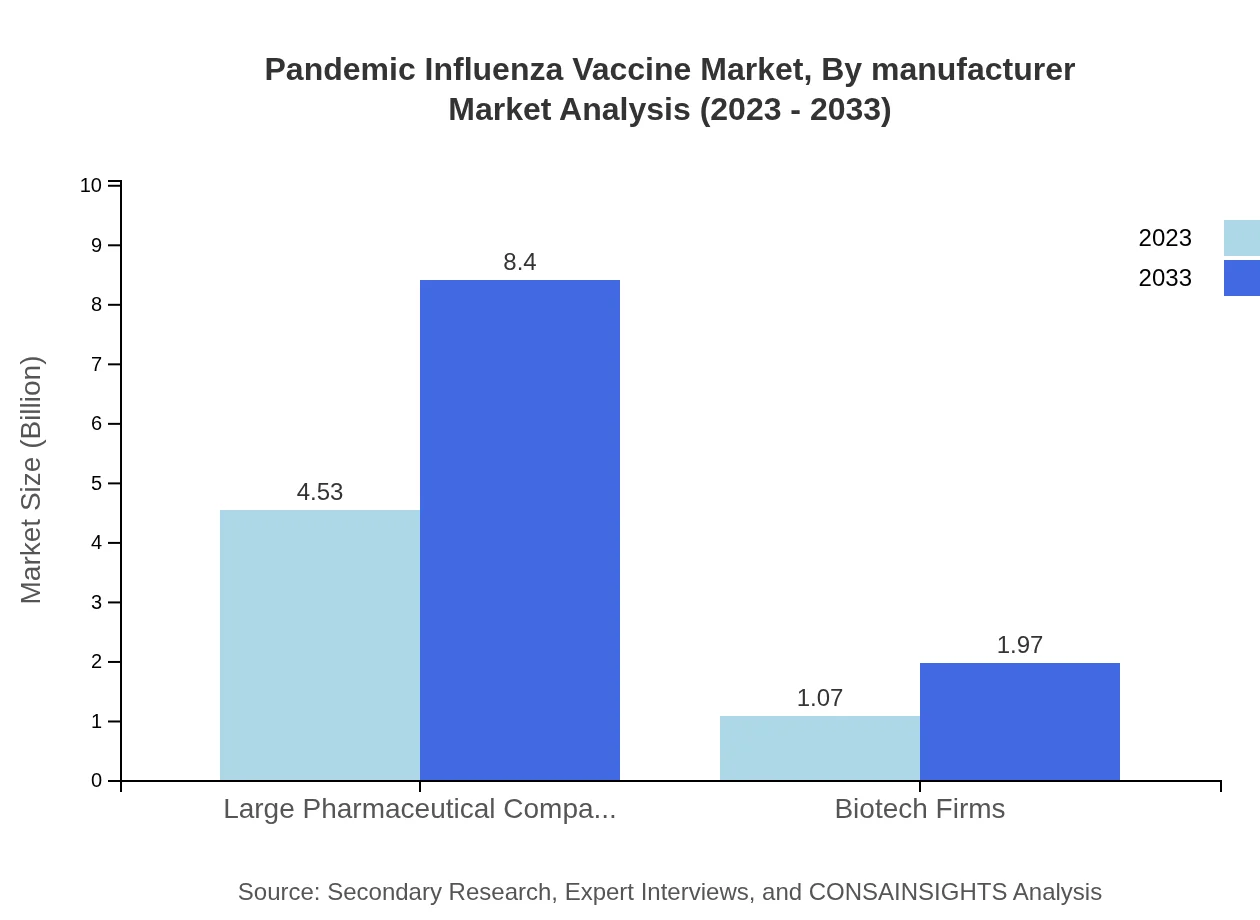
Pandemic Influenza Vaccine Market Analysis By Manufacturer
Analysis of the manufacturer segment indicates that Large Pharmaceutical Companies lead with a substantial $4.53 billion in 2023, expected to reach $8.40 billion by 2033, holding a marked market share of 80.98%. Smaller Biotech Firms, however, are gradually increasing their market presence from $1.07 billion to $1.97 billion within the same time frame.
Pandemic Influenza Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pandemic Influenza Vaccine Industry
Pfizer Inc.:
A leading pharmaceutical company known for its innovative vaccines developed using advanced recombinant technology, Pfizer has a strong portfolio in pandemic preparedness.Sanofi Pasteur:
With a historical emphasis on vaccine research and development, Sanofi Pasteur is a significant player in providing vaccines for widespread use, including pandemic influenza.GlaxoSmithKline (GSK):
GSK specializes in developing both preventive and therapeutic vaccines, playing a vital role in combating influenza pandemics through comprehensive vaccine programs.Moderna, Inc.:
Emerging as a frontrunner in mRNA vaccine technology, Moderna focuses on innovative approaches to rapidly develop impactful vaccines, including those for influenza.Merck & Co.:
Merck is recognized for a diverse portfolio in therapeutic and preventive vaccines, continuously investing in research to enhance the efficacy and safety of influenza vaccines.We're grateful to work with incredible clients.









FAQs
What is the market size of pandemic Influenza Vaccine?
The pandemic influenza vaccine market is projected to reach approximately $5.6 billion by 2033, with a compound annual growth rate (CAGR) of 6.2% from 2023 to 2033.
What are the key market players or companies in this pandemic Influenza Vaccine industry?
Key players in the pandemic influenza vaccine industry include large pharmaceutical companies and biotech firms that dominate with a combined market share of approximately 100%, focusing on human use vaccines.
What are the primary factors driving the growth in the pandemic Influenza Vaccine industry?
Key growth drivers for the pandemic influenza vaccine market include government initiatives, increasing awareness of vaccine benefits, advancements in vaccine technology, and the ongoing threat of influenza pandemics.
Which region is the fastest Growing in the pandemic Influenza Vaccine?
The fastest-growing regions in the pandemic influenza vaccine market are Europe and North America. By 2033, Europe’s market will reach $2.79 billion, and North America’s market will grow to $3.75 billion.
Does ConsaInsights provide customized market report data for the pandemic Influenza Vaccine industry?
Yes, ConsaInsights offers customized market report data specific to the pandemic influenza vaccine industry, tailored to meet the unique needs of clients.
What deliverables can I expect from this pandemic Influenza Vaccine market research project?
From this pandemic-influenza-vaccine market research project, you can expect detailed market analysis reports, segment-wise insights, regional breakdowns, and future growth forecasts.
What are the market trends of pandemic Influenza Vaccine?
Current trends in the pandemic influenza vaccine market include a shift towards inactivated and recombinant vaccines, with inactivated vaccines holding a significant market share, reflecting advancements in vaccine formulation.