Prostate Cancer Diagnostics And Therapy Market Report
Published Date: 31 January 2026 | Report Code: prostate-cancer-diagnostics-and-therapy
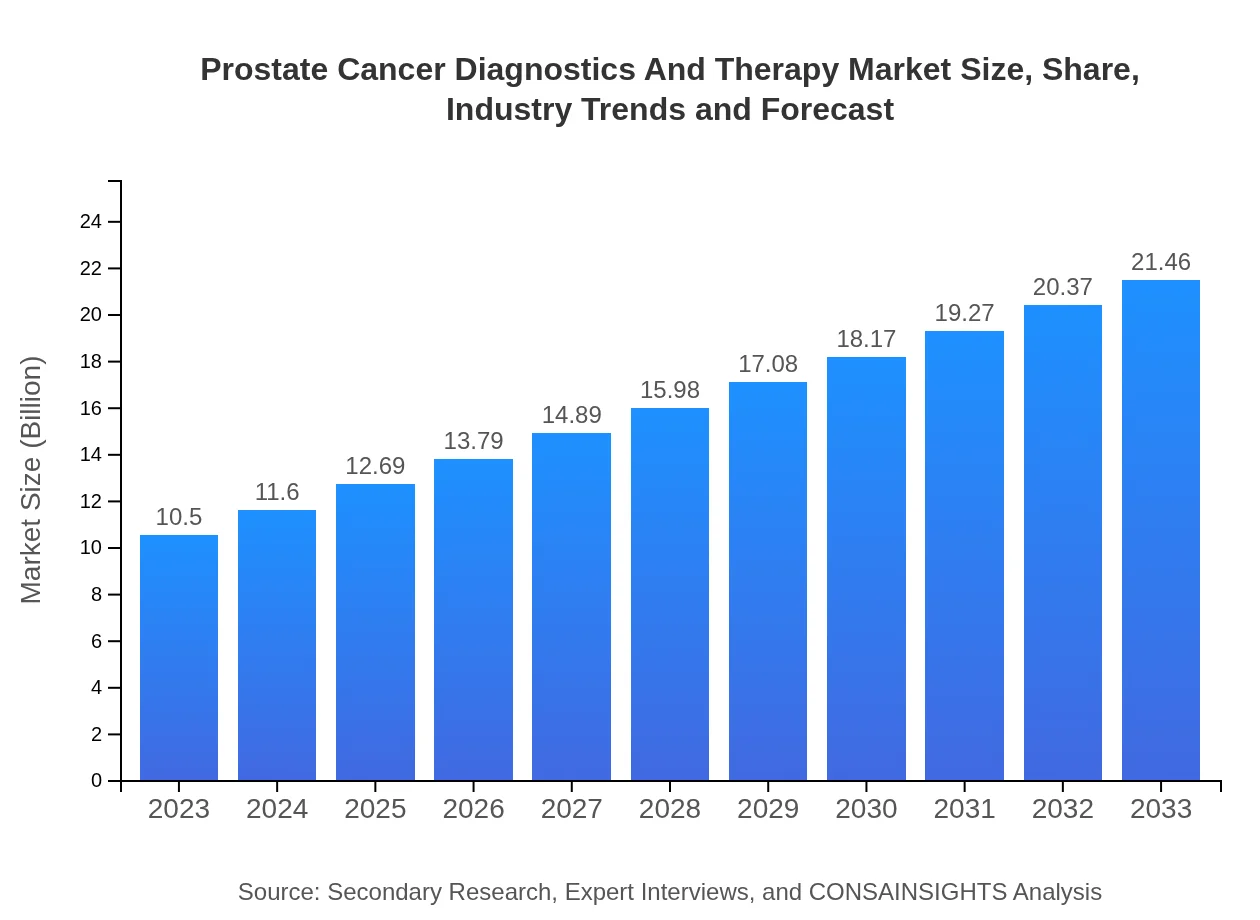
Prostate Cancer Diagnostics And Therapy Market Size, Share, Industry Trends and Forecast to 2033
This report provides an insightful analysis of the Prostate Cancer Diagnostics and Therapy market, covering market size, growth projections, and trends from 2023 to 2033. It also includes a breakdown of the market by region and segment, offering valuable insights for industry stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $21.46 Billion |
| Top Companies | AbbVie Inc., Bristol-Myers Squibb, Thermo Fisher Scientific Inc., Roche Holding AG, Johnson & Johnson |
| Last Modified Date | 31 January 2026 |
Prostate Cancer Diagnostics And Therapy Market Overview
Customize Prostate Cancer Diagnostics And Therapy Market Report market research report
- ✔ Get in-depth analysis of Prostate Cancer Diagnostics And Therapy market size, growth, and forecasts.
- ✔ Understand Prostate Cancer Diagnostics And Therapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Prostate Cancer Diagnostics And Therapy
What is the Market Size & CAGR of Prostate Cancer Diagnostics And Therapy market in 2023?
Prostate Cancer Diagnostics And Therapy Industry Analysis
Prostate Cancer Diagnostics And Therapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Prostate Cancer Diagnostics And Therapy Market Analysis Report by Region
Europe Prostate Cancer Diagnostics And Therapy Market Report:
The European prostate cancer diagnostics and therapy market is expected to grow from $3.03 billion in 2023 to $6.20 billion by 2033, driven mainly by aging populations and increasing incidences of prostate cancer in various countries.Asia Pacific Prostate Cancer Diagnostics And Therapy Market Report:
In 2023, the Asia Pacific market for prostate cancer diagnostics and therapy is valued at approximately $1.98 billion, with projections to reach about $4.04 billion by 2033. Key factors driving growth include increasing healthcare expenditure, a burgeoning geriatric population, and rising awareness about prostate cancer among men.North America Prostate Cancer Diagnostics And Therapy Market Report:
North America represents the largest market share, valued at about $3.99 billion in 2023, with a forecast to reach $8.16 billion by 2033. This growth is fueled by advanced healthcare infrastructure, high adoption rates of new technologies, and significant investments in prostate cancer research.South America Prostate Cancer Diagnostics And Therapy Market Report:
The South American market is anticipated to grow from $0.28 billion in 2023 to about $0.56 billion by 2033. Increased public health initiatives aimed at early detection and treatment options are anticipated to enhance market growth in this region.Middle East & Africa Prostate Cancer Diagnostics And Therapy Market Report:
The market in the Middle East and Africa is projected to increase from $1.22 billion in 2023 to $2.50 billion by 2033. This growth can be attributed to improving healthcare systems and increased investments in diagnostic services.Tell us your focus area and get a customized research report.
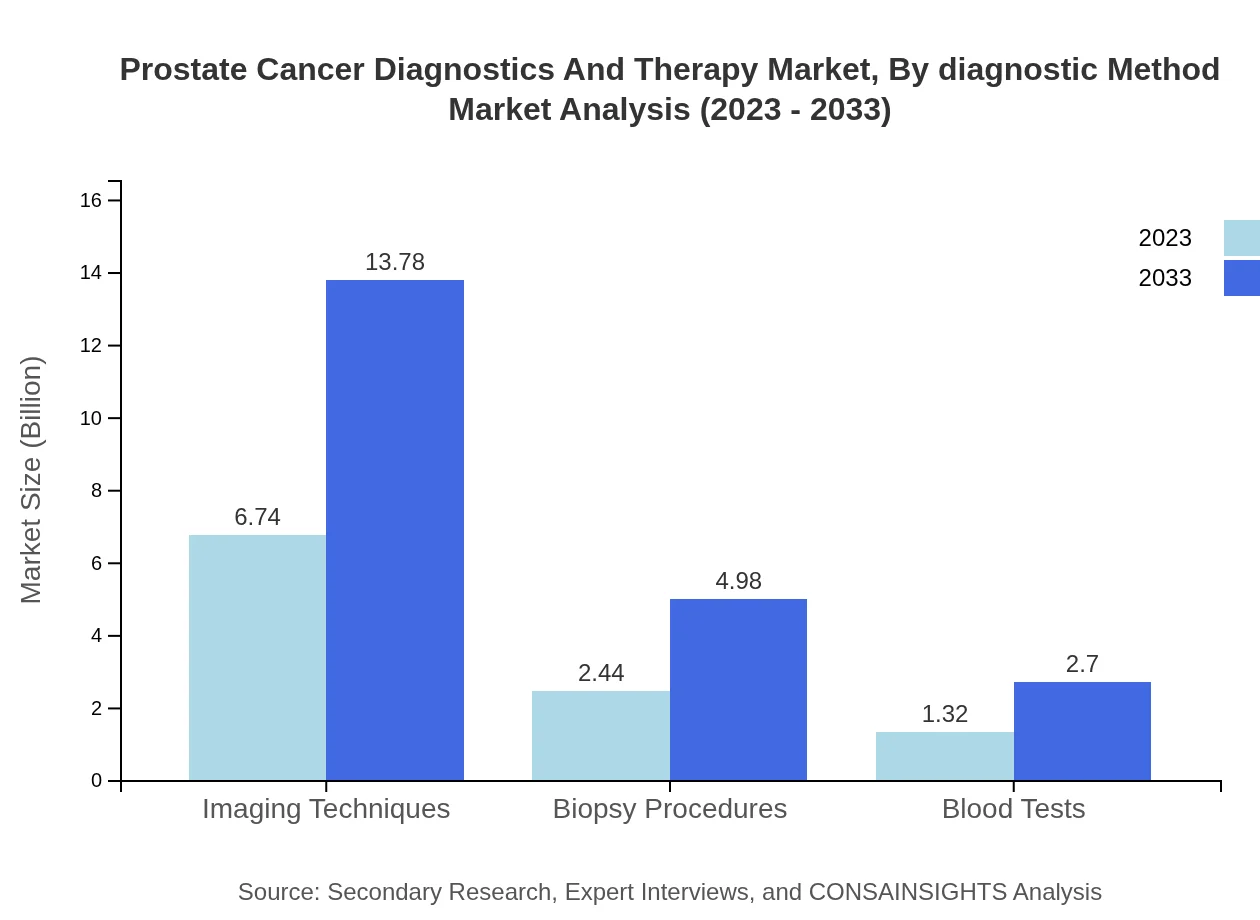
Prostate Cancer Diagnostics And Therapy Market Analysis By Diagnostic Method
The Prostate Cancer Diagnostics market consists of various diagnostic methods including imaging techniques, biopsy procedures, and blood tests. In 2023, imaging techniques lead the diagnostics segment with a market size of 6.74 billion, expected to grow to 13.78 billion by 2033. Biopsy procedures and blood tests follow with significant market shares, emphasizing the necessity of accurate diagnostics in effective treatment planning.
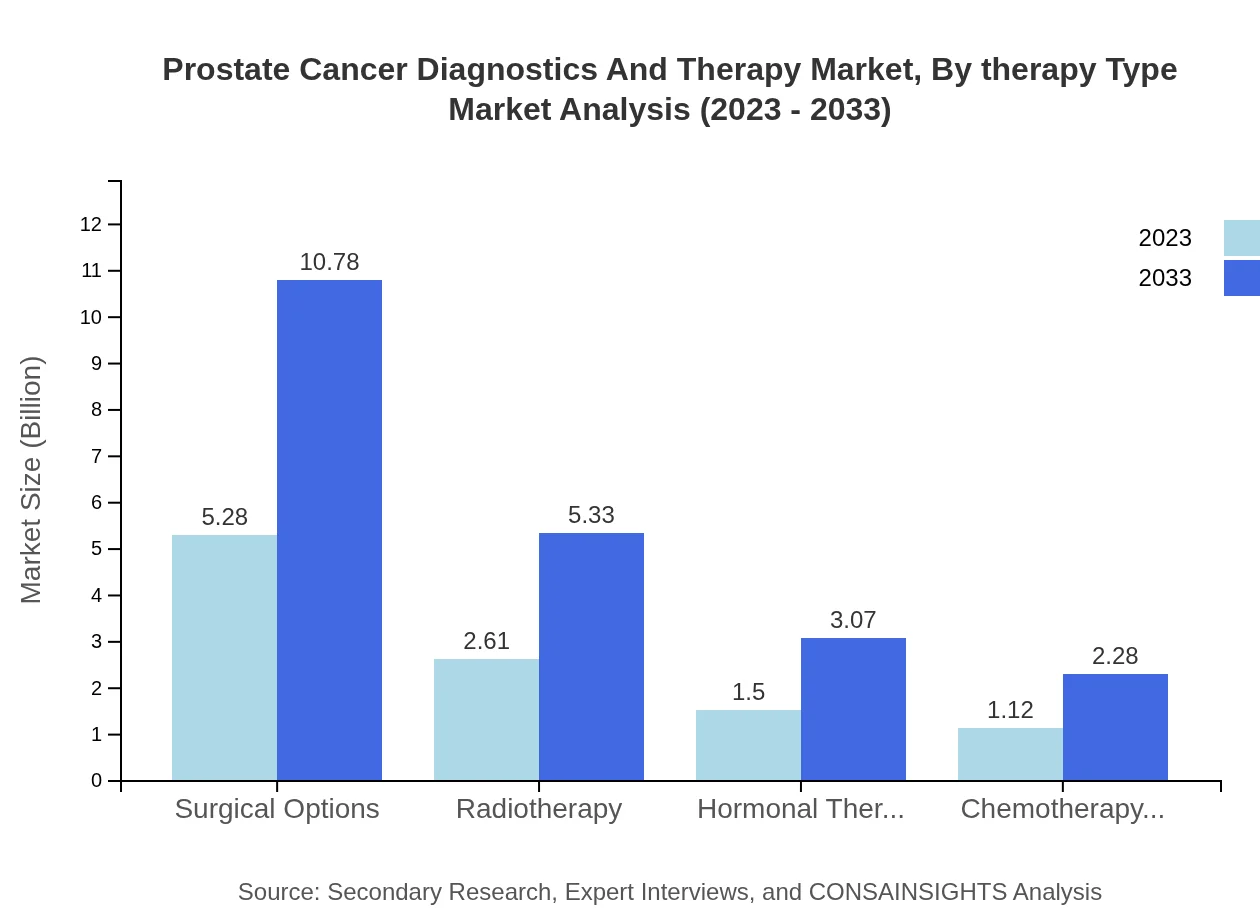
Prostate Cancer Diagnostics And Therapy Market Analysis By Therapy Type
The Prostate Cancer Therapy market is segmented into surgical options, radiotherapy, hormonal therapy, and chemotherapy. Surgical options currently capture the largest share at 50.24% of the market, valued at about 5.28 billion in 2023 and projected to reach 10.78 billion by 2033. Other therapies such as chemotherapy and hormonal therapy are also expected to experience robust growth due to technological advancements and improved treatment protocols.
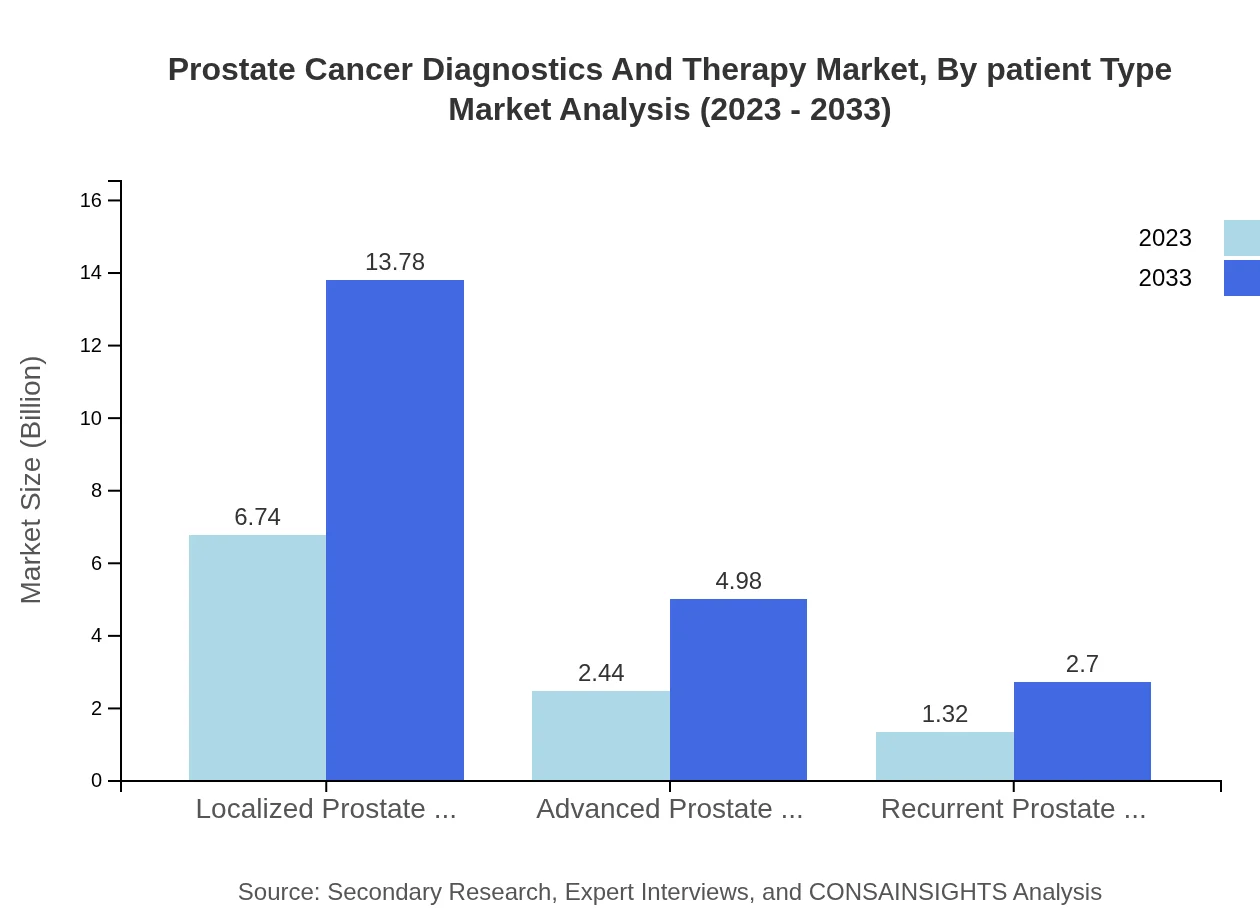
Prostate Cancer Diagnostics And Therapy Market Analysis By Patient Type
The market is categorized into localized, advanced, and recurrent prostate cancer types. Localized prostate cancer holds the majority market share at 64.21% in 2023 and is expected to remain significant due to the high success rates of early interventions. Advanced prostate cancer and recurrent prostate cancer follow, necessitating the development of targeted therapies to improve patient outcomes.
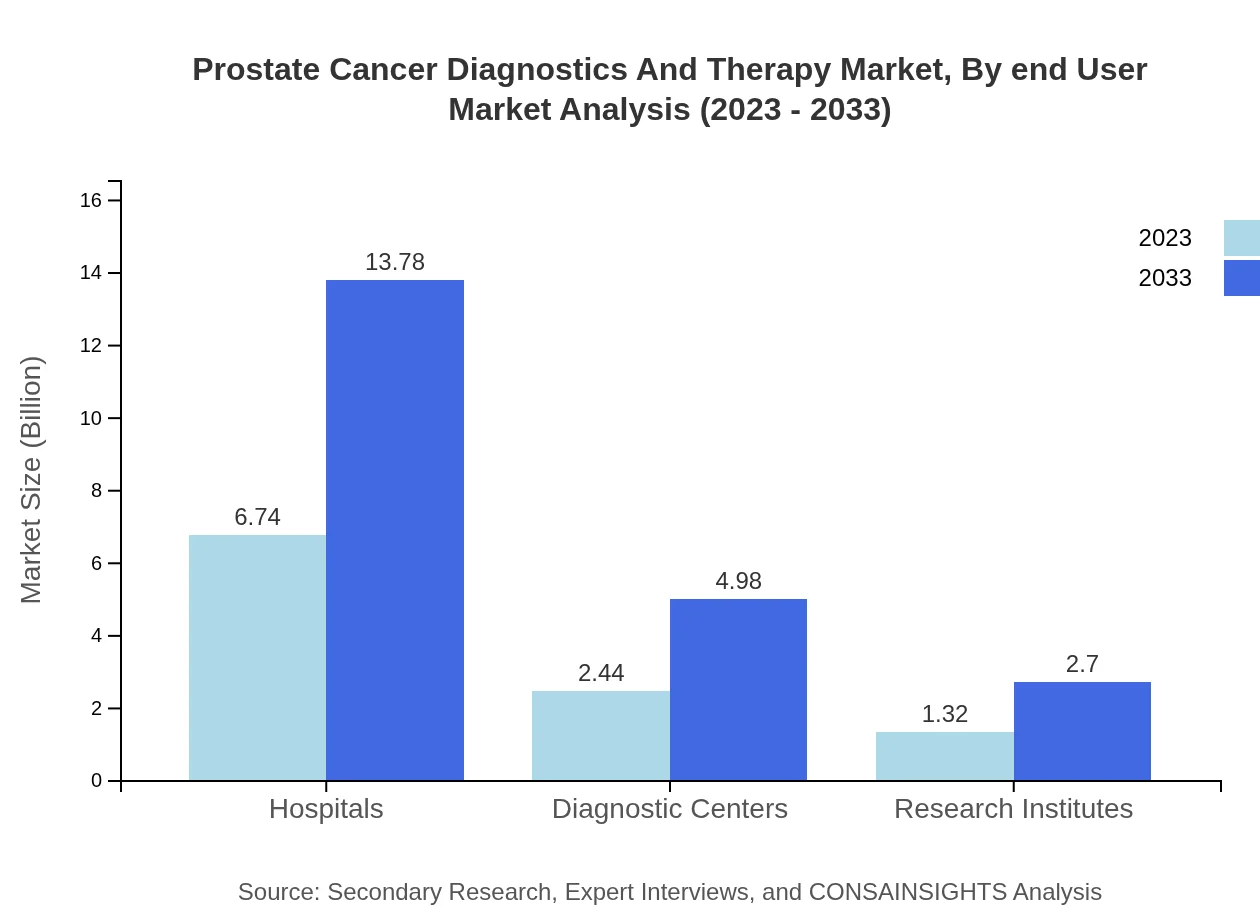
Prostate Cancer Diagnostics And Therapy Market Analysis By End User
End-users of prostate cancer diagnostics and therapy include hospitals, diagnostic centers, and research institutes. Hospitals account for a significant portion of the market, reflecting their role as the primary healthcare providers. Diagnostic centers and research institutes play essential roles in providing precise diagnostics and advancing treatment methodologies, respectively.
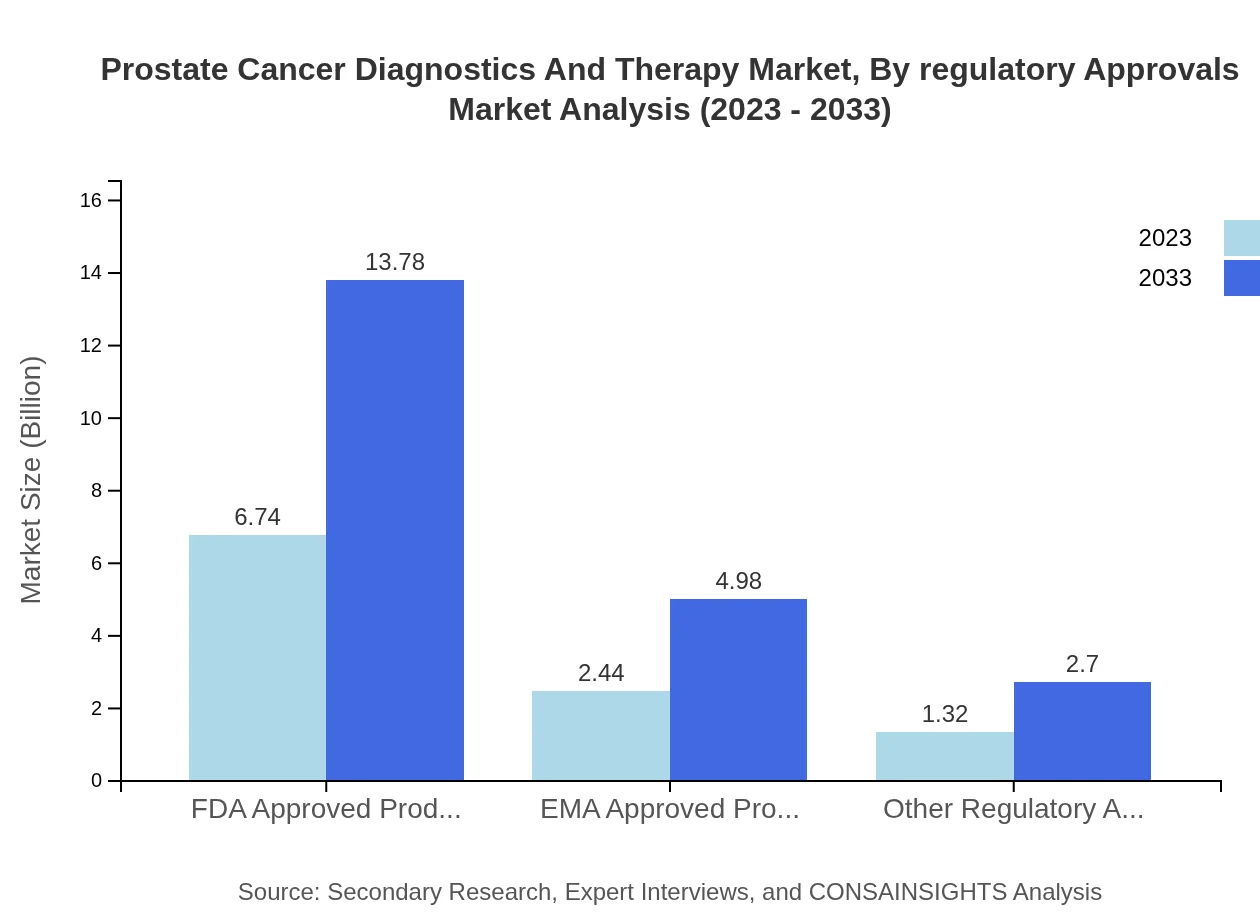
Prostate Cancer Diagnostics And Therapy Market Analysis By Regulatory Approvals
Regulatory approvals play a vital role in bringing prostate cancer diagnostics and therapies to market. Products approved by the FDA and EMA dominate this segment, comprising substantial market shares. The emphasis on regulatory compliance is critical for maintaining product safety and efficacy, thereby influencing market dynamics significantly.
Prostate Cancer Diagnostics And Therapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Prostate Cancer Diagnostics And Therapy Industry
AbbVie Inc.:
AbbVie is a global biopharmaceutical company and a key player in the prostate cancer market, offering innovative therapies such as prostate inhibitors and advanced medical devices.Bristol-Myers Squibb:
Bristol-Myers Squibb focuses on discovering and developing innovative cancer therapies. Their contributions to prostate cancer treatments are vital for enhancing patient outcomes.Thermo Fisher Scientific Inc.:
Thermo Fisher is instrumental in providing cutting-edge diagnostic equipment and solutions to accurately detect prostate cancer, thus playing a significant role in the early diagnosis.Roche Holding AG:
Roche is heavily involved in the research and development of advanced diagnostics and therapeutic options for prostate cancer, aiming to deliver effective treatment solutions.Johnson & Johnson:
Johnson & Johnson plays a major role in developing medical devices and therapies for prostate cancer, enabling improved treatment practices in healthcare facilities.We're grateful to work with incredible clients.









FAQs
What is the market size of prostate cancer diagnostics and therapy?
The prostate cancer diagnostics and therapy market is projected to reach approximately $10.5 billion by 2033, with a compound annual growth rate (CAGR) of 7.2% from 2023 onwards.
What are the key market players or companies in the prostate cancer diagnostics and therapy industry?
The key players in this market include major pharmaceutical companies, diagnostic device manufacturers, and healthcare providers that offer innovative solutions in prostate cancer diagnostics and therapeutic interventions.
What are the primary factors driving the growth in the prostate cancer diagnostics and therapy industry?
Factors contributing to market growth include increased awareness of prostate cancer, advancements in diagnostic technologies, rising healthcare expenditure, and an aging population that is more susceptible to prostate-related health issues.
Which region is the fastest Growing in the prostate cancer diagnostics and therapy?
The fastest-growing region in the prostate cancer diagnostics and therapy market is North America, expected to grow from $3.99 billion in 2023 to $8.16 billion by 2033, driven by advancements in treatment options and healthcare accessibility.
Does ConsaInsights provide customized market report data for the prostate cancer diagnostics and therapy industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the prostate cancer diagnostics and therapy industry, ensuring clients receive targeted insights that align with their strategic objectives.
What deliverables can I expect from this prostate cancer diagnostics and therapy market research project?
Deliverables from this market research include comprehensive reports, detailed segment analyses, market forecasts, competitive landscape assessments, and actionable insights tailored to your business goals within the prostate cancer diagnostics and therapy space.
What are the market trends of prostate cancer diagnostics and therapy?
Current trends include a shift towards personalized medicine, increased utilization of minimally invasive surgical techniques, and the integration of artificial intelligence in diagnostics, shaping the future of prostate cancer treatment and management.